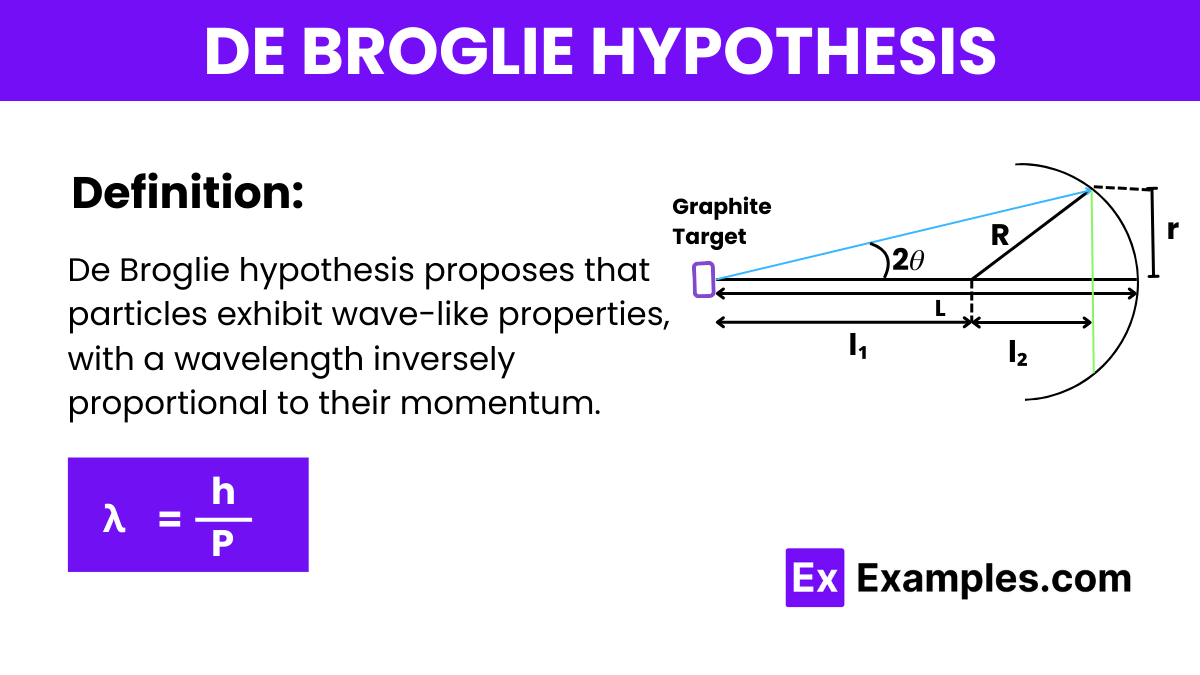
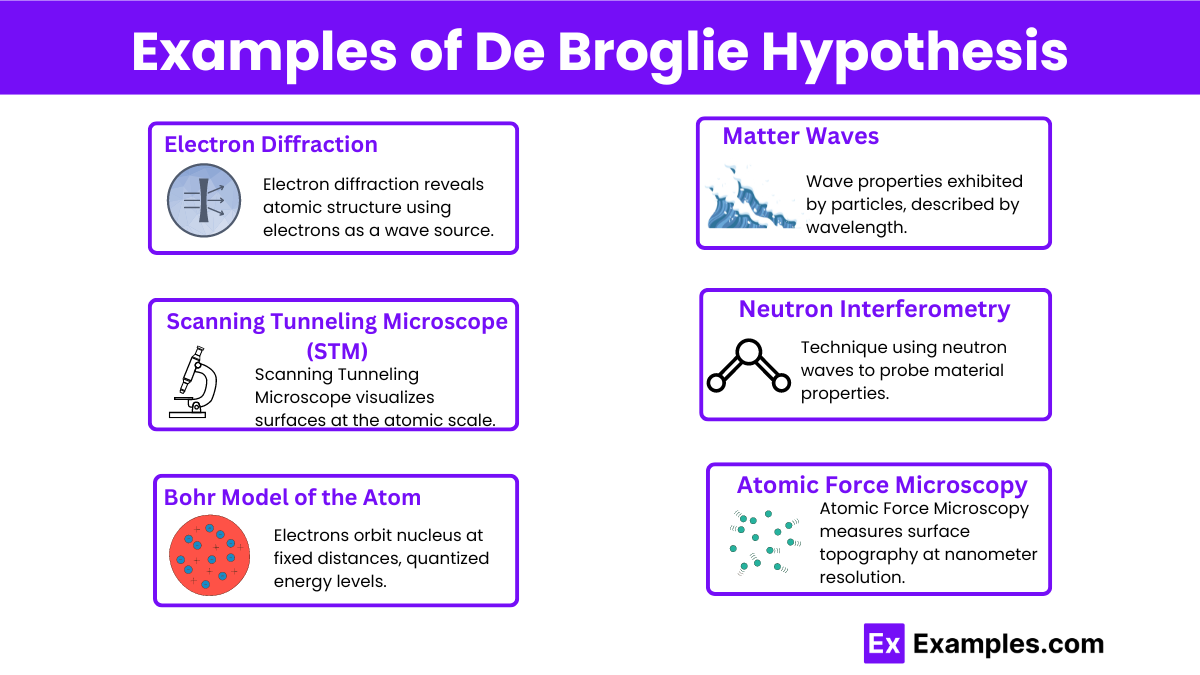
Which of the following statements best describes the de Broglie hypothesis?
Particles exhibit only wave-like properties.
Particles exhibit only particle-like properties.
Particles exhibit both wave-like and particle-like properties.
Particles exhibit neither wave-like nor particle-like properties.