Which law describes the conservation of mass and energy in nuclear reactions?
Law of Conservation of Momentum
Law of Conservation of Charge
Law of Conservation of Mass-Energy
Law of Conservation of Angular Momentum

Nuclear physics, a captivating branch of physics, delves into the intricate laws that govern the components and behavior of an atom’s nucleus. Essential to understanding the fundamental aspects of our universe, these laws of physics explore how protons and neutrons within the nucleus interact through forces like the strong nuclear force, which is pivotal in holding the nucleus together. This area of physics not only sheds light on the building blocks of matter but also plays a crucial role in fields ranging from medicine to energy production, offering profound insights into the power and subtleties of nature at an atomic level.
The Law of Conservation of Mass-Energy is fundamental in nuclear physics. It asserts that the total amount of mass and energy in an isolated system remains constant. During nuclear reactions, mass can be converted into energy and vice versa. This concept famously encapsulated in Einstein’s equation E=mc². This transformation is crucial in processes like nuclear fusion, where light nuclei combine at high temperatures to form heavier nuclei, releasing substantial energy.
The Law of Conservation of Charge dictates that the total electric charge in an isolated system must remain constant before and after any reaction. In nuclear reactions, this law ensures that the sum of the charges of all particles involved does not change. For example, if a neutron in a nucleus decays into a proton, an electron, and an anti-neutrino (beta decay), the overall charge is conserved with the proton and electron charges balancing each other.
The Law of Conservation of Nucleon Number states that the total number of nucleons (protons and neutrons) in a nuclear reaction remains unchanged. This law is critical for maintaining nuclear stability and is observable in reactions like alpha decay, where a nucleus emits an alpha particle (two protons and two neutrons). It resulting in a new nucleus with four fewer nucleons, but the total number of nucleons in the system remains the same.
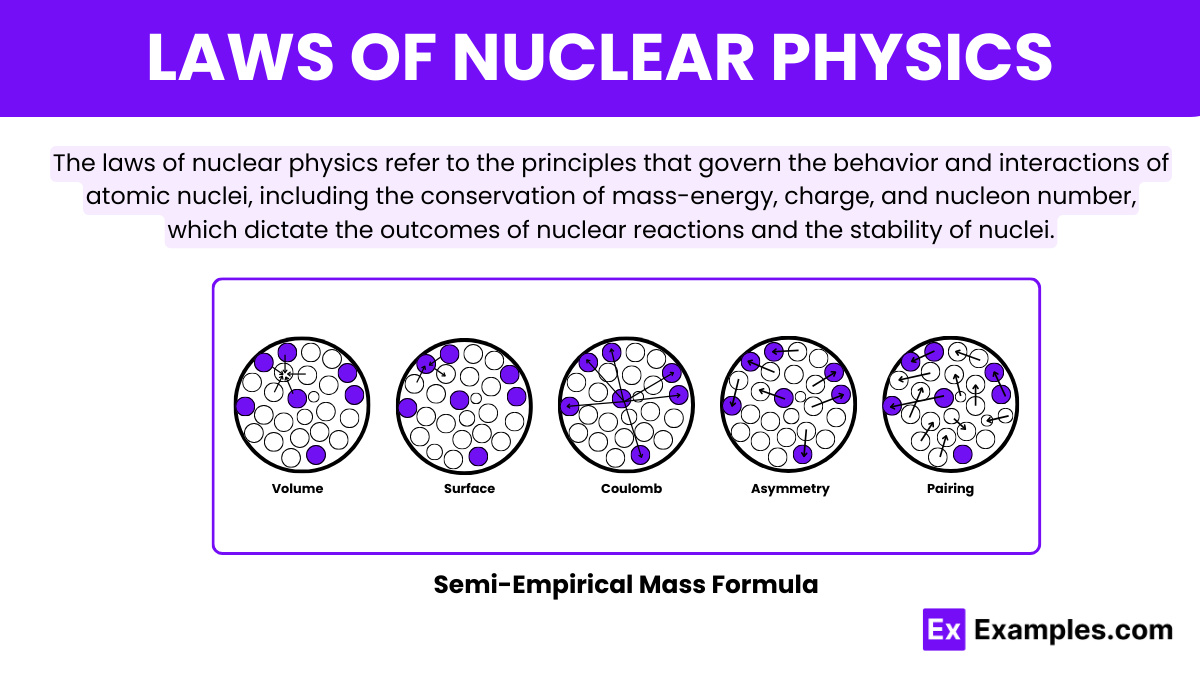
The Semi-Empirical Mass Formula (SEMF) is also known as the Weizsäcker Formula. It serves as a critical theoretical model in nuclear physics for approximating the binding energy of atomic nuclei. Developed by Carl Friedrich von Weizsäcker in 1935. This formula integrates several physical insights and empirical data to predict the stability, mass, and energy dynamics of nuclei. The SEMF is particularly useful in explaining why certain isotopes exhibit more stability and in predicting the energies involved in nuclear reactions such as fission and fusion.
The formula comprises several key terms, each reflecting different nuclear forces and interactions. The Volume Term suggests that binding energy is proportional to the number of nucleons. It acknowledging that each nucleon interacts primarily with its nearest neighbors. The Surface Term adjusts for nucleons at the nuclear surface who have fewer neighbors and thus lesser binding energy. Thus effectively scaling with the nucleus’s surface area. Coulomb Term accounts for the electrostatic repulsion among protons, diminishing the binding energy proportional to the proton count and inversely to the nucleus radius.
Further refinements include the Asymmetry Term, which deals with the stability effects of having unequal numbers of protons and neutrons, favoring those nuclei where these numbers are close. The Pairing Term adds stability calculations for nuclei with even numbers of protons and neutrons, reflecting observed extra stability in such structures. Collectively, these terms allow the SEMF to make quick yet accurate predictions about nuclear properties, facilitating advanced studies and practical applications in nuclear energy and weaponry without extensive quantum mechanical computations.
The decay law describes the rate at which unstable atomic nuclei or particles lose energy by emitting radiation, quantifying decay through a half-life.
No, nuclear fusion does not violate the law of conservation of energy. It converts mass into energy, adhering to energy conservation principles.
The three nuclear conservation laws are: conservation of mass-energy, conservation of charge, and conservation of nucleon number.
Laws of Nuclear Physics – Definition, Conservation of Laws, Semi-Empirical Mass Formula , Applications
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Which law describes the conservation of mass and energy in nuclear reactions?
Law of Conservation of Momentum
Law of Conservation of Charge
Law of Conservation of Mass-Energy
Law of Conservation of Angular Momentum
What does the Law of Conservation of Charge state in nuclear physics?
The total charge before and after a nuclear reaction is the same
The total mass before and after a nuclear reaction is the same
The total energy before and after a nuclear reaction is the same
The total momentum before and after a nuclear reaction is the same
Which law is applied to determine the stability of nuclei?
Law of Conservation of Momentum
Law of Conservation of Energy
Nuclear Binding Energy Law
Law of Conservation of Angular Momentum
What does the Law of Radioactive Decay describe?
The process of nuclear fusion
The rate at which unstable nuclei lose energy by emitting radiation
The process of nuclear fission
The conservation of mass in nuclear reactions
In nuclear physics, what does the Law of Conservation of Nucleon Number state?
The total number of nucleons (protons and neutrons) remains constant in a nuclear reaction
The total energy remains constant in a nuclear reaction
The total mass remains constant in a nuclear reaction
The total charge remains constant in a nuclear reaction
Which principle explains the balance between nuclear forces and electrostatic repulsion in the nucleus?
Pauli Exclusion Principle
Heisenberg Uncertainty Principle
Liquid Drop Model
Shell Model
What is the main concept of the Pauli Exclusion Principle in nuclear physics?
Two protons cannot occupy the same energy level
Two neutrons cannot occupy the same energy level
No two identical fermions can occupy the same quantum state simultaneously
All nucleons can occupy the same quantum state
What does the Heisenberg Uncertainty Principle state in the context of nuclear physics?
The position and momentum of a particle can be precisely determined simultaneously
The position and momentum of a particle cannot both be precisely determined at the same time
The energy and time of a particle can be precisely determined simultaneously
The charge and mass of a particle can be precisely determined simultaneously
Which law governs the emission of alpha, beta, and gamma radiation from unstable nuclei?
Law of Conservation of Mass-Energy
Law of Radioactive Decay
Law of Conservation of Momentum
Law of Nuclear Stability
What does the Law of Conservation of Momentum state in nuclear reactions?
The total energy remains constant in a nuclear reaction
The total charge remains constant in a nuclear reaction
The total momentum before and after a nuclear reaction is the same
The total mass remains constant in a nuclear reaction
Before you leave, take our quick quiz to enhance your learning!

