Molecular Structure
Molecular structure is the arrangement of atoms within a molecule, and it’s a fundamental concept in chemistry. Understanding molecular structure helps us know how atoms bond together to form different substances. Each molecule has a unique structure that determines its properties and behavior in chemical reactions. By studying molecular structure, we can predict how molecules will interact, combine, and change, making it essential for understanding everything from simple compounds to complex biological systems. In chemistry, knowing the molecular structure allows us to explore and explain the vast world of chemical reactions and materials around us.
What is Molecular Structure?
Types of Molecular Structure
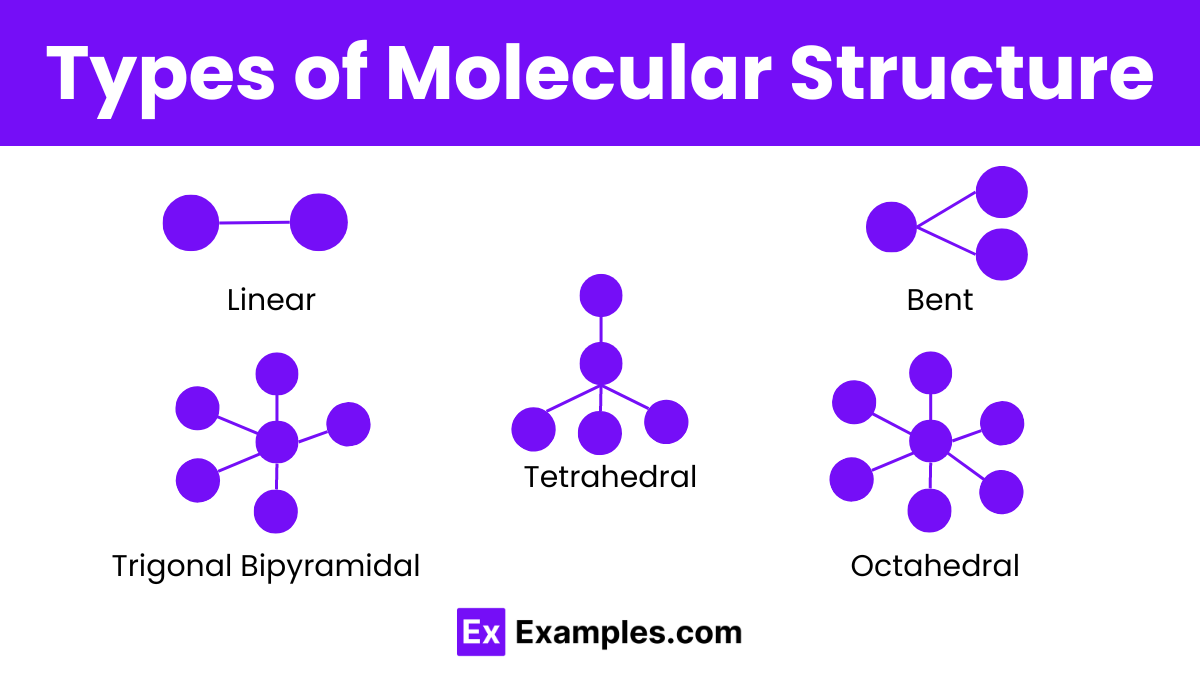
Linear Structure
In a linear molecular structure, atoms align in a straight line. This structure usually occurs when there are only two atoms or when the central atom has no lone pairs of electrons. An example of a linear molecule is carbon dioxide (CO₂), where the carbon atom is in the center with oxygen atoms on either side, forming a straight line.
Bent or Angular Structure
A bent or angular structure happens when the central atom has lone pairs of electrons that push the bonded atoms away, creating a bent shape. Water (H₂O) is a common example, with the oxygen atom in the center and hydrogen atoms at an angle, forming a bent shape.
Tetrahedral Structure
A tetrahedral structure has a central atom bonded to four atoms, which form the vertices of a tetrahedron. This three-dimensional shape is common in many molecules, like methane (CH₄), where the carbon atom is in the center, and hydrogen atoms occupy the four corners.
Trigonal Bipyramidal Structure
In a trigonal bipyramidal structure, five atoms surround a central atom: three in a plane (equatorial positions) and two above and below the plane (axial positions). Phosphorus pentachloride (PCl₅) is an example, with phosphorus in the center and chlorine atoms arranged in this specific pattern.
Octahedral Structure
An octahedral structure features a central atom surrounded by six atoms positioned at the corners of an octahedron. Sulfur hexafluoride (SF₆) illustrates this structure, where sulfur is at the center, and fluorine atoms are evenly distributed around it.
Examples of Molecular Structure
- Water (H₂O)
- Carbon Dioxide (CO₂)
- Methane (CH₄)
- Boron Trifluoride (BF₃)
- Ammonia (NH₃)
- Sulfur Hexafluoride (SF₆)
- Phosphorus Pentachloride (PCl₅)
- Ethylene (C₂H₄)
- Acetylene (C₂H₂)
- Hydrogen Cyanide (HCN)
- Nitrogen (N₂)
- Oxygen (O₂)
- Sulfur Dioxide (SO₂)
- Nitric Acid (HNO₃)
- Formaldehyde (CH₂O)
- Carbon Tetrachloride (CCl₄)
- Xenon Tetrafluoride (XeF₄)
- Benzene (C₆H₆)
- Phosphine (PH₃)
- Sulfur Trioxide (SO₃)
- Hydrogen Peroxide (H₂O₂)
- Chlorine Trifluoride (ClF₃)
- Xenon Hexafluoride (XeF₆)
- Dichloromethane (CH₂Cl₂)
- Ethanol (C₂H₅OH)
- Nitrous Oxide (N₂O)
- Ozone (O₃)
- Phosgene (COCl₂)
- Tetrafluoromethane (CF₄)
- Iodine Pentafluoride (IF₅)
How to Determine the Molecular Structure
- Draw the Lewis Structure
- Count Valence Electrons: Add up the total number of valence electrons for all atoms in the molecule.
- Arrange Atoms: Place the least electronegative atom in the center (usually) and arrange the other atoms around it.
- Distribute Electrons: Place electron pairs between atoms to form bonds. Distribute remaining electrons to complete the octet (or duet for hydrogen) for each atom.
- Determine the Electron Pair Geometry
- Count Electron Pairs: Count the bonding pairs (shared electrons) and lone pairs (unshared electrons) around the central atom.
- Predict Shape: Use the Valence Shell Electron Pair Repulsion (VSEPR) theory to predict the arrangement of electron pairs, minimizing repulsion between them.
- Predict the Molecular Shape
- Based on the VSEPR theory, determine the geometry of the molecule:
- 2 Electron Pairs: Linear (e.g., CO₂)
- 3 Electron Pairs: Trigonal planar (e.g., BF₃)
- 4 Electron Pairs: Tetrahedral (e.g., CH₄)
- 5 Electron Pairs: Trigonal bipyramidal (e.g., PCl₅)
- 6 Electron Pairs: Octahedral (e.g., SF₆)
- Adjust the shape considering lone pairs:
- Bent: If there are lone pairs, the shape will adjust (e.g., H₂O is bent due to 2 lone pairs).
- Trigonal Pyramidal: For example, NH₃ has one lone pair and three bonding pairs.
- Based on the VSEPR theory, determine the geometry of the molecule:
- Consider Multiple Bonds and Resonance
- Double and triple bonds count as one region of electron density.
- If the molecule has resonance structures, consider the average position of atoms.
- Check for Polarity
- Determine if the molecule is polar or nonpolar by checking the symmetry and the electronegativity differences between atoms.
Bond Angles of Molecular Structure
The bond angles in molecular structures are determined by the arrangement of electron pairs around the central atom, which in turn is influenced by the VSEPR (Valence Shell Electron Pair Repulsion) theory.
| Molecular Structure | Bond Angle(s) |
|---|---|
| Linear | 180° |
| Bent | <120° (3 electron groups) <109.5° (4 electron groups) |
| Trigonal Planar | 120° |
| Trigonal Pyramidal | <109.5° |
| Tetrahedral | 109.5° |
| Trigonal Bipyramidal | 90°, 120° |
| Octahedral | 90° |
| Square Planar | 90° |
How to Draw a Molecular Structure
Drawing a molecular structure involves several steps that include determining the number of valence electrons, arranging the atoms, and using the VSEPR theory to predict the shape.
- Count the Total Number of Valence Electrons
- Add up the valence electrons from all the atoms in the molecule. For ions, add or subtract electrons based on the charge (add for negative charge, subtract for positive charge).
- Write the Skeletal Structure
- Place the least electronegative atom in the center (usually). Hydrogen and halogens are usually placed on the outside.
- Connect the central atom to surrounding atoms with single bonds.
- Distribute Remaining Electrons
- Subtract the electrons used in the single bonds from the total number of valence electrons.
- Distribute the remaining electrons to complete the octets of the outer atoms first (except for hydrogen, which needs only 2 electrons).
- Complete the Octet for the Central Atom
- If there are remaining electrons after completing the octets of the outer atoms, place them on the central atom.
- If the central atom does not have an octet, form double or triple bonds as necessary by sharing lone pairs from outer atoms.
- Determine the Molecular Geometry Using VSEPR Theory
- Count the number of bonding pairs (shared electrons) and lone pairs (unshared electrons) around the central atom.
- Use the VSEPR theory to predict the shape based on the repulsion between electron pairs.
- Draw the Final Structure
- Sketch the structure with correct bond angles and shapes.
- Indicate lone pairs if necessary.
Importance of Molecular Structure
- Determines Physical Properties: Molecular structure influences properties such as boiling point, melting point, solubility, and density.
- Affects Chemical Reactivity: The arrangement of atoms and electrons affects how molecules interact and react with each other.
- Predicts Molecular Behavior: Understanding the structure helps predict how molecules will behave in different environments.
- Guides Drug Design: In pharmaceuticals, molecular structure is crucial for designing effective and specific drugs.
- Influences Biological Function: The shape of molecules like proteins and DNA is essential for their function in biological systems.
- Impacts Material Properties: The structure of polymers and other materials determines their mechanical, electrical, and thermal properties.
- Enables Spectroscopic Analysis: Techniques like NMR and IR spectroscopy rely on molecular structure to identify substances.
- Facilitates Understanding of Molecular Interactions: Knowledge of structure aids in understanding how molecules interact in processes like catalysis and enzyme activity.
- Supports Environmental Chemistry: Understanding molecular structure helps predict the behavior of pollutants and their interactions in the environment.
- Aids in Synthesis: Knowing the structure is essential for designing synthetic pathways in chemistry.
Application of Molecular Structure
- Pharmaceuticals: Designing and developing new drugs based on the specific molecular targets.
- Biochemistry: Understanding enzyme-substrate interactions and protein folding.
- Materials Science: Designing new materials with specific properties for technology and industry.
- Nanotechnology: Creating nanoscale devices and materials with unique properties.
- Environmental Science: Predicting the behavior and fate of chemicals in the environment.
- Forensic Science: Analyzing substances in crime scene investigations.
- Agriculture: Developing pesticides and fertilizers that are more effective and environmentally friendly.
- Food Science: Understanding the molecular basis of flavors and nutrition.
- Polymer Chemistry: Designing polymers with specific properties for various applications.
- Medicinal Chemistry: Modifying molecules to improve their therapeutic properties.
- Chemical Engineering: Optimizing reactions and processes in industrial applications.
- Cosmetics: Formulating products with desirable properties and performance.
- Energy Storage: Developing better batteries and fuel cells.
- Catalysis: Designing catalysts that increase the efficiency of chemical reactions.
- Toxicology: Assessing the molecular basis of toxicity in chemicals.
- Synthetic Chemistry: Creating new molecules with specific functions.
- Structural Biology: Determining the structures of biological macromolecules.
- Quality Control: Ensuring the consistency and safety of products.
- Genetics: Understanding the molecular basis of genetic information.
- Medical Diagnostics: Developing diagnostic tools based on molecular interactions.
What is Actual Molecular Structure?
Actual molecular structure refers to the three-dimensional arrangement of atoms within a molecule, determining its shape, properties, and reactivity.
What is a Simple Molecular Structure?
A simple molecular structure consists of a small number of atoms bonded together, like H₂O (water) or CH₄ (methane), forming easily understood shapes.
What is Molecular Structure for Kids?
Molecular structure for kids is the way atoms connect and form shapes, like how water is shaped like a “V” with oxygen in the middle and hydrogen on the sides.
Do Humans Have a Molecular Structure?
Yes, humans have a molecular structure. Our bodies are composed of complex molecules like proteins, DNA, and lipids, determining our biological functions.
What is the Famous Molecular Structure?
The famous molecular structure is the DNA double helix, discovered by Watson and Crick, which carries genetic information in living organisms.
Does Everything Have a Molecular Structure?
Yes, everything has a molecular structure. All matter is made up of molecules with specific arrangements of atoms, defining their properties.
How Many Molecular Structures Are There?
There are countless molecular structures, varying with the number and arrangement of atoms and the types of bonds they form.
What is the Molecular Shape of Water
The molecular shape of water (H₂O) is bent or V-shaped, with a bond angle of approximately 104.5° due to two hydrogen atoms bonded to oxygen.
What Can Be Used to Predict Molecular Structure?
VSEPR theory (Valence Shell Electron Pair Repulsion) can be used to predict molecular structure by considering electron pair repulsion around the central atom.
Is Trigonal Planar Polar or Nonpolar?
Trigonal planar molecules can be polar or nonpolar, depending on the symmetry and the electronegativity differences between the central atom and surrounding atoms.