What is the chemical formula of phosphoric acid?
H₂SO₄
HNO₃
H₃PO₄
HCl

Phosphoric acid is a key player in the world of chemistry. It is a substance you might not see, but it plays a huge role in things we use regularly. This acid known scientifically as H₃PO₄. It is not just any acid. It’s a superhero in the chemistry lab, making its mark in everything from fertilizers that help our food grow to the soft drinks we enjoy. It’s a clear, colorless liquid with a slightly syrupy consistency that can also appear in a solid form as crystals at lower temperatures. Phosphoric acid is fascinating because it shows us how it is part of our daily lives, from the food we eat to the products we use.
| Property | Value |
|---|---|
| Formula | H₃PO₄ |
| Hill Formula | H₃O₄P |
| Name | Phosphoric acid |
| Alternate Names | Orthophosphoric acid, Evits, Sonac, White phosphoric acid |
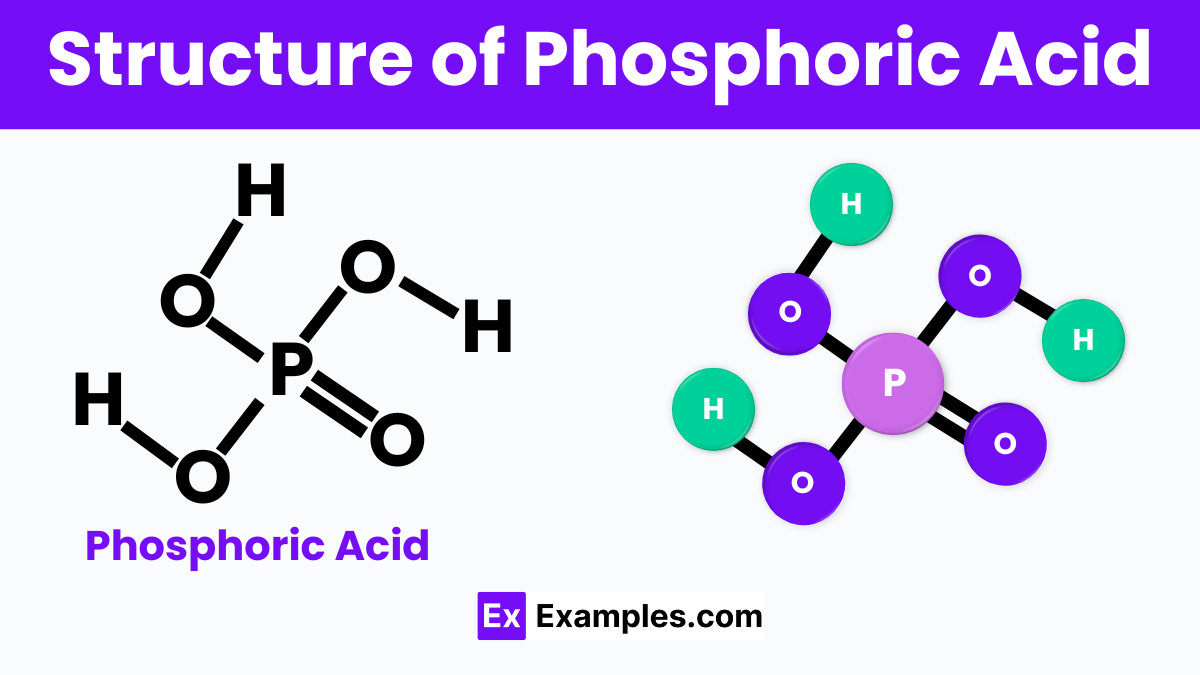
Phosphoric Acid, known to scientists as H₃PO₄, has a fascinating structure that makes it quite unique. Picture a central phosphorus atom, like the sun in our solar system, surrounded by four oxygen atoms, similar to planets. Three of these oxygen atoms hold onto a hydrogen atom each, like moons orbiting their planets. This special arrangement gives Phosphoric Acid its acidic properties, allowing it to participate in various chemical reactions, from fertilizing plants to flavoring your favorite soft drinks. Its structure not only explains why it’s an acid but also how it can form strong bonds with other substances, making it incredibly useful in both nature and industry.
Preparing Phosphoric Acid, a key ingredient in many everyday products, involves a chemical process that starts with a mineral called phosphorus rock. The most common method is called the wet process. Here’s how it works: the phosphorus rock is mixed with sulfuric acid, leading to a chemical reaction that produces Phosphoric Acid. The equation for this reaction looks something like this:
This equation shows how calcium phosphate (from the rock), sulfuric acid, and water mix together to create Phosphoric Acid, gypsum (a by-product), and hydrogen fluoride. The Phosphoric Acid produced through this process is not only crucial for making fertilizers but also plays a significant role in food and beverage industries, adding that tangy taste to some of your favorite drinks.
| Property | Description |
|---|---|
| Appearance | Clear, colorless syrupy liquid or a solid (when pure) |
| Odor | Odorless |
| Chemical Formula | H3PO4 |
| Melting Point | 42.35°C (solid form) |
| Boiling Point | 158°C (decomposes) |
| Density | 1.885 g/cm³ (at 85% concentration) |
| Solubility | Completely soluble in water, forming a mildly acidic solution |
| pH | Highly acidic, with a pH of around 2 in its common concentrations |
| Property | Value |
|---|---|
| CAS registry number | 7664-38-2 |
| Beilstein number | 1921286 |
| PubChem compound ID | 1004 |
| PubChem substance ID | 24861303 |
| SMILES identifier | OP(=O)(O)O |
| InChI identifier | InChI=1/H3O4P/c1-5(2,3)4/h(H3,1,2,3,4)/f/h1-3H |
| RTECS number | TB6300000 |
| MDL number | MFCD00011340 |
| Property | Value |
|---|---|
| NFPA health rating | 2 |
| NFPA fire rating | 0 |
| NFPA reactivity rating | 0 |
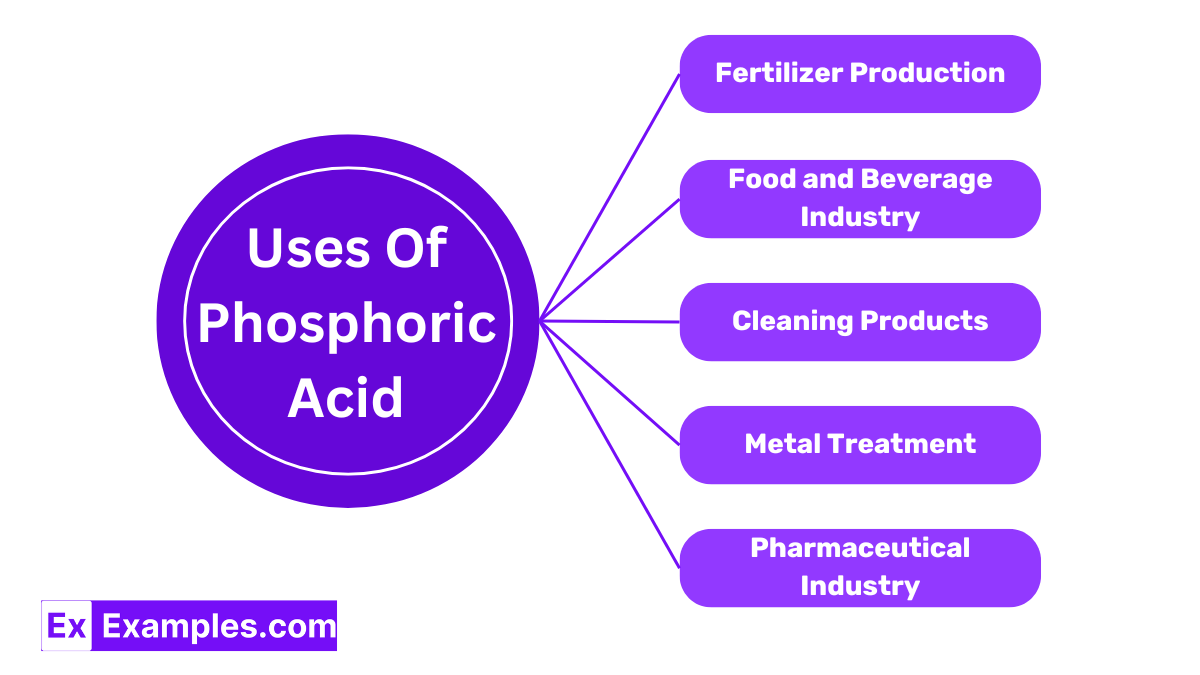
Phosphoric Acid is a key ingredient in manufacturing fertilizers, especially those that supply phosphate, a crucial nutrient for plant growth. It helps to promote root development and increase crop yields.
In the food industry, Phosphoric Acid is used as a food additive to acidify foods and beverages like colas, providing a tangy or sour taste. It also serves as a preservative to prolong the shelf life of various products.
Due to its ability to dissolve rust and mineral deposits, Phosphoric Acid is commonly found in household cleaning products. It’s particularly effective in toilet bowl cleaners and products designed to clean hard water stains.
In metalworking, Phosphoric Acid is used to prepare metal surfaces for coating or painting. It helps to create a protective layer that prevents rust and improves paint adhesion.
Phosphoric Acid is also used in dentistry as an etching solution to clean and roughen the surfaces of teeth before filling them. Additionally, it’s employed in various medical syrups to adjust pH levels.
This acid plays a role in water treatment facilities, where it is used to remove impurities and contaminants from water, making it safe for drinking.
In pharmaceutical manufacturing, Phosphoric Acid is utilized as a reagent and pH adjuster in various medications, ensuring they are effective and safe for consumption.
Phosphoric Acid can be harmful, causing digestive issues and reducing bone density if consumed excessively.
Phosphoric Acid is a moderately strong acid, capable of donating three hydrogen ions in aqueous solutions.
Drinking Phosphoric Acid in high concentrations can cause severe irritation to the gastrointestinal tract, leading to pain and nausea.
The lethal dose of Phosphoric Acid varies, but ingestion of highly concentrated forms can be fatal due to chemical burns and organ damage.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula of phosphoric acid?
H₂SO₄
HNO₃
H₃PO₄
HCl
What is the molecular weight of phosphoric acid?
98 g/mol
60 g/mol
83 g/mol
97 g/mol
Phosphoric acid is commonly used in which industry?
Textile
Food and beverage
Automobile
Aerospace
Phosphoric acid is a type of:
Carboxylic acid
Sulfuric acid
Mineral acid
Organic acid
Which of the following is a common use of phosphoric acid?
Fertilizer production
Plastic manufacturing
Textile dyeing
Battery production
What is the pH of a 1M solution of phosphoric acid?
1
2
7
14
Phosphoric acid can be described as:
Monoprotic
Diprotic
Triprotic
Tetraprotic
Which of the following is a property of phosphoric acid?
Strong oxidizing agent
Highly flammable
Corrosive to metals
Non-reactive with bases
What is the role of phosphoric acid in rust removal?
Reducing agent
Oxidizing agent
Chelating agent
Acid-base neutralizer
What is the IUPAC name of phosphoric acid?
Orthophosphoric acid
Hydroxylphosphoric acid
Phosphorous acid
Pyrophosphoric acid
Before you leave, take our quick quiz to enhance your learning!

