What is the fundamental principle of Boyle's Law?
Pressure is directly proportional to volume
Pressure is inversely proportional to volume
Pressure is directly proportional to temperature
Pressure is inversely proportional to temperature

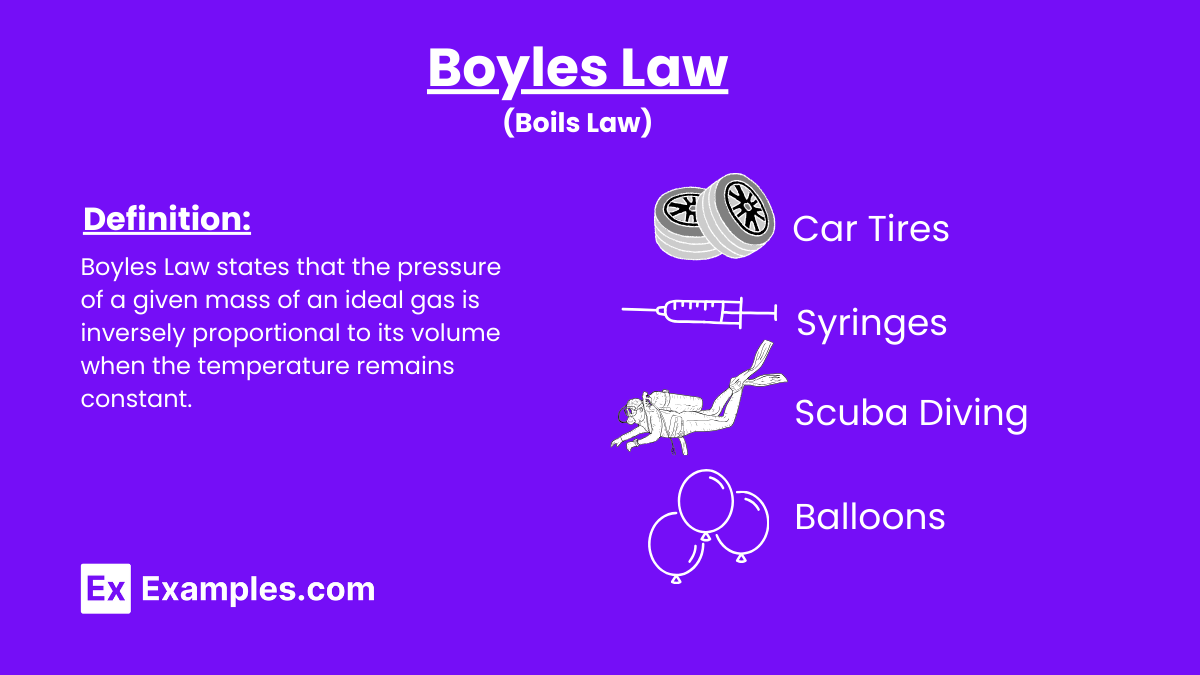
Boyle’s Law is a fundamental principle in the field of physics, specifically within the realm of Laws of Thermodynamics and gas laws. This law states that the pressure of a given mass of an ideal gas is inversely proportional to its volume when the temperature remains constant. In simpler terms, if you increase the volume of a gas container, the pressure of the gas decreases, provided the temperature does not change.
Formally expressed, Boyle’s Law is: 𝑃×𝑉=constant, where P represents the pressure of the gas, and 𝑉 denotes the volume. This relationship is a cornerstone in the laws of physics, showcasing how gases behave under varying pressures and volumes, and is crucial in applications ranging from respiratory physiology to engineering.
The formula for Boyle’s Law is expressed as:
Where:
This equation demonstrates that for a given amount of gas at a constant temperature, the product of the pressure and volume remains constant, even as the individual values of pressure and volume change
Boyle’s Law states that the pressure and volume of a gas have an inverse relationship when temperature is held constant. Here is a concise derivation:
Starting Point: Assume the gas is ideal and that its temperature remains constant.
Ideal Gas Law: For a constant amount of gas at constant temperature, the ideal gas law 𝑃𝑉=𝑛𝑅𝑇 simplifies because 𝑛 (number of moles), 𝑅 (gas constant), and 𝑇 (temperature) are constants.
Constant Product: With 𝑛𝑅𝑇 being constant, 𝑃𝑉 is also constant. Thus, if the volume 𝑉 increases, the pressure 𝑃 must decrease proportionally to maintain the equality, and vice versa.
Mathematical Expression: This relationship can be expressed as: 𝑃₁𝑉₁=𝑃₂𝑉₂ Here, 𝑃₁ and 𝑉₁ are initial pressure and volume, and 𝑃₂ and 𝑉₂ are the final pressure and volume under constant temperature conditions.
This derivation succinctly captures Boyle’s Law, emphasizing the inverse proportionality between pressure and volume at constant temperature for an ideal gas.
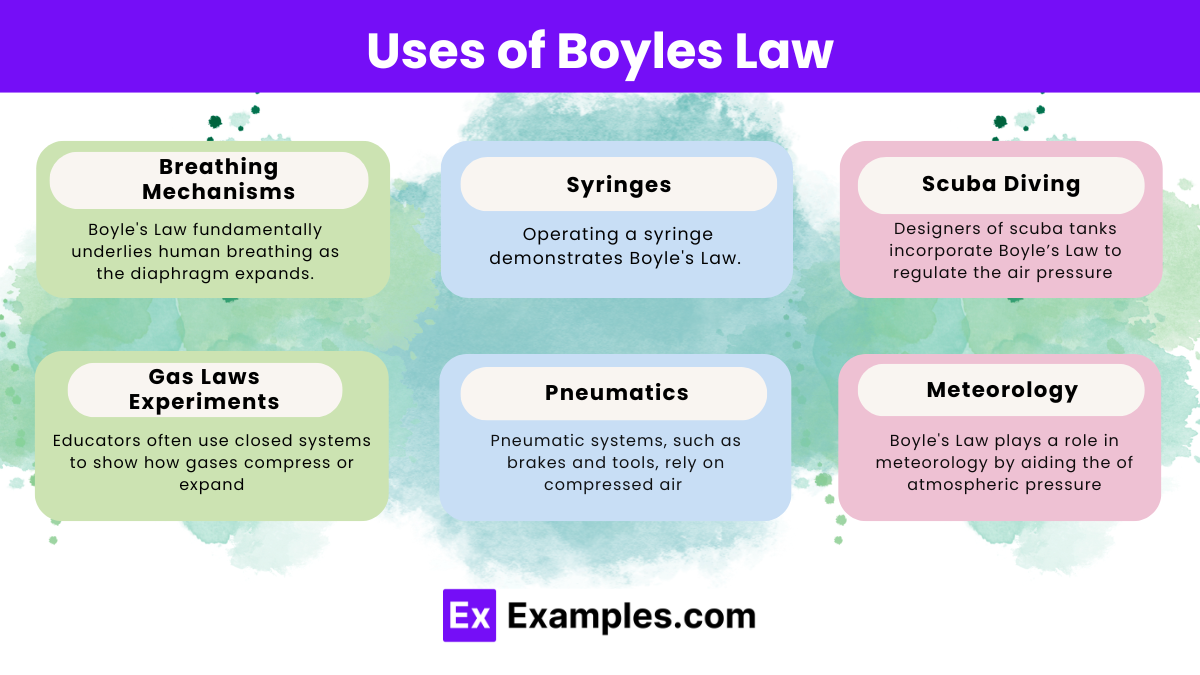
Boyle’s Law describes the inverse relationship between the pressure and volume of a gas at constant temperature and finds application across various fields:
Boyle’s Law plays a vital role across various fields and situations, illustrating the inverse relationship between pressure and volume at constant temperature:
Irish scientist Robert Boyle discovered Boyle’s Law in the 17th century.
Pressure is typically measured in atmospheres (atm) or Pascals (Pa), and volume in liters (L) or cubic meters (m³).
It describes the behavior of ideal gases when temperature is held constant.
Volume decreases proportionally to pressure increase, maintaining the product of pressure and volume constant.
It’s a good approximation for real gases at low pressures and high temperatures but may deviate at extremes.
It explains atmospheric pressure changes, such as in weather systems and altimeter readings in aviation.
It’s fundamental for understanding gas behavior in reactions, especially in ideal gas law calculations.
It’s vital in respiratory therapy equipment design, where pressure and volume adjustments are crucial for patient care.
Pressure increases proportionally to volume decrease, maintaining the product of pressure and volume constant.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the fundamental principle of Boyle's Law?
Pressure is directly proportional to volume
Pressure is inversely proportional to volume
Pressure is directly proportional to temperature
Pressure is inversely proportional to temperature
Boyle's Law can be mathematically expressed as:
PV = nRT
P/V = constant
PV = constant
P + V = constant
If the volume of a gas is doubled while the temperature remains constant, what happens to the pressure according to Boyle's Law?
It doubles
It remains the same
It halves
It becomes zero
According to Boyle's Law, if the volume of a gas is decreased, what happens to its pressure?
It increases
It decreases
It remains constant
It remains constant
Boyle's Law is valid under which condition?
Constant temperature
Constant pressure
Constant volume
Constant mass
If a gas occupies a volume of 4 liters at a pressure of 2 atm, what will be its volume when the pressure is increased to 4 atm, assuming temperature remains constant?
1 liter
2 liter
3 liter
4 liter
In Boyle's Law, what does the term "constant" refer to?
The product of pressure and volume
The sum of pressure and volum
The ratio of pressure and volume
The difference between pressure and volume
Boyle's Law can be graphically represented by plotting:
Pressure vs. Temperature
Volume vs. Temperature
Pressure vs. Volume
Volume vs. Time
A gas is compressed from 10 liters to 5 liters. If the initial pressure was 1 atm, what is the final pressure?
0.5 atm
1 atm
2 atm
3 atm
Boyle's Law is a part of which larger set of gas laws?
Charles's Law
Ideal Gas Law
Avogadro's Law
Gay-Lussac's Law
Before you leave, take our quick quiz to enhance your learning!

