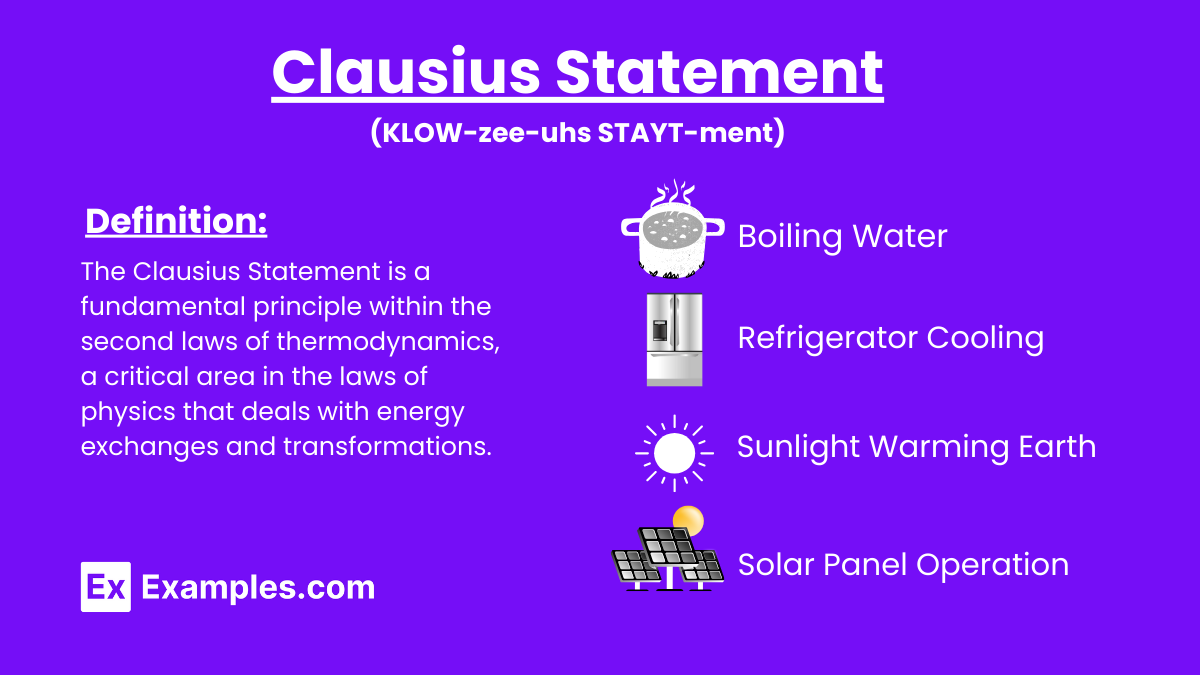
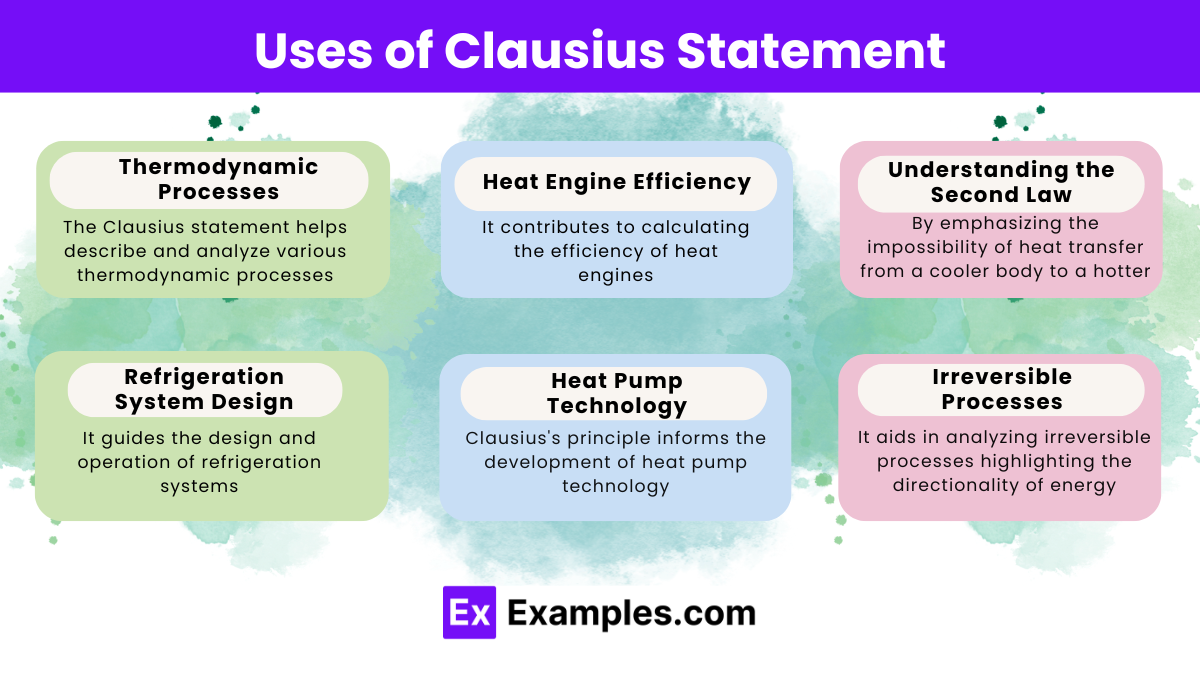
What does the Clausius statement of the second law of thermodynamics state?
Energy cannot be created or destroyed
Heat cannot spontaneously flow from a colder body to a hotter body
The entropy of an isolated system always increases
Work can be converted into heat