What is the Joule-Thomson effect?
A temperature change in a gas when it expands without doing work
A pressure change in a liquid when it is compressed
A temperature change in a solid when it is heated
A volume change in a gas when it is heated

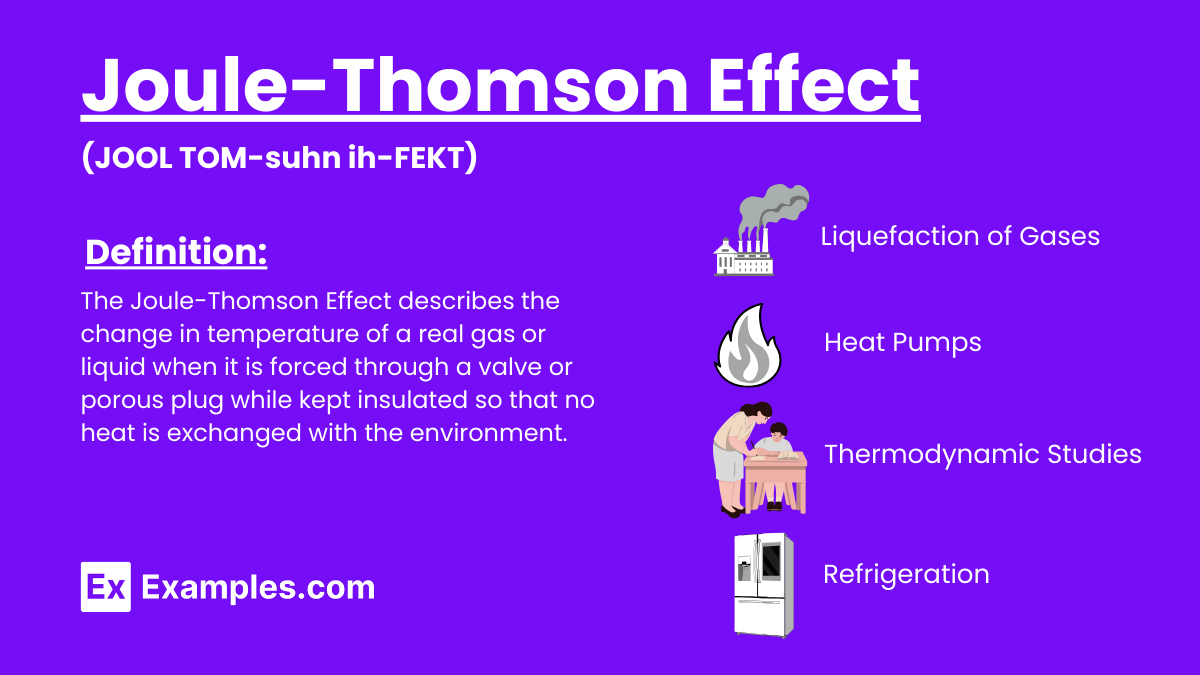
The Joule-Thomson Effect describes the change in temperature of a real gas or liquid when it is forced through a valve or porous plug while kept insulated so that no heat is exchanged with the environment. This effect is significant in thermodynamics and has practical applications in refrigeration and liquefaction of gases.
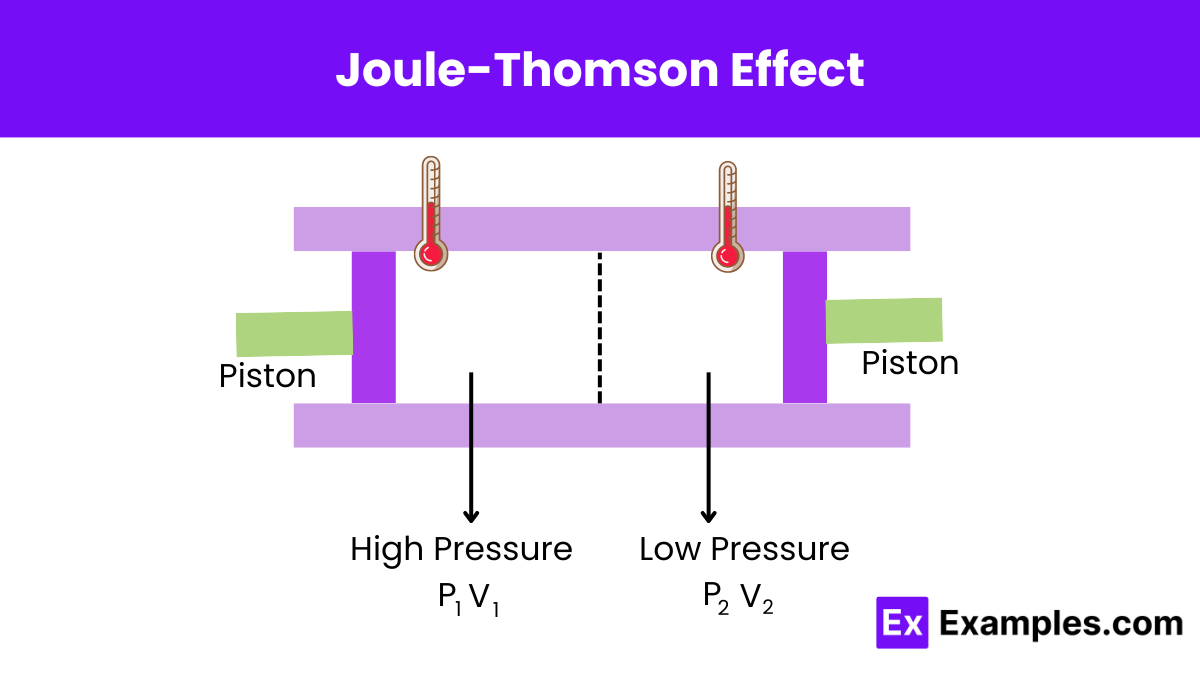
According to thermodynamic principles, we can best understand the Joule-Thomson effect by considering a gas packet moving against the flow through a restriction. The upstream gas must perform work to push the gas packet forward through this restriction. This work equals the product of the gas packet’s volume and the upstream pressure.
𝑊₁ = 𝑉ₚₐ꜀ₖₑₜ ₁ ×𝑃₁
As the gas packet moves through the restriction, it needs to create space by displacing some of the downstream gas. This involves performing work that is the product of the packet volume and the downstream pressure.
𝑊₂ = 𝑉ₚₐ꜀ₖₑₜ ₂×𝑃₂
Due to varying compressibility effects, real gases do not have equal work done upstream and downstream. Since this depressurization is an adiabatic process, the system does not exchange heat with the surroundings. Thus, the change in internal energy must adhere to the first law of thermodynamics.
𝑈₂−𝑈₁=𝑊₁−𝑊₂
Gas molecules experience both repulsive and attractive forces (Van der Waals forces) as they are in constant motion. When the gas pressure is reduced, the average distance between the molecules increases, causing the attractive forces to dominate for many gases at room temperature. This dominance results in an increase in potential energy.
Most real gases require more work downstream at ambient temperatures due to compressibility effects.
𝑃₁×𝑉₁<𝑃₂×𝑉₂
This indicates that the internal energy decreases when the gas moves through the restriction.
Generally, for many real gases, the temperature decreases during a pressure drop. However, this is not universal for all gases and conditions. Since the process is isenthalpic (constant enthalpy), the temperature can either decrease or increase depending on how the internal energy changes to maintain constant enthalpy.
The Joule-Thomson coefficient 𝜇ⱼₜ is defined as the rate of change of temperature 𝑇 with respect to pressure P during a constant enthalpy process:
𝜇ⱼₜ = (∂𝑇/∂𝑃)𝐻
The following table explains when the Joule-Thomson effect cools or warms a real gas:
| If the gas temperature is | then 𝜇ⱼₜ is | since ∂P is | thus ∂𝑇 must be | So the gas |
|---|---|---|---|---|
| above the inversion temperature | negative | always negative | positive | warms |
| below the inversion temperature | positive | always negative | negative | cools |
The Joule-Thomson coefficient can be derived using thermodynamic relationships and is defined as the change in temperature during an isenthalpic process (constant enthalpy) due to a pressure drop:
𝜇 = (∂𝑇/∂𝑃)𝐻
Applying the cyclic rule of partial derivatives:
(∂𝑇/∂𝑃)ₕ(∂𝑃/∂𝐻)ₜ(∂𝐻/∂𝑇)ₚ = −1
Since:
(∂𝑃∂𝐻)ₜ = −(∂𝐻∂𝑃)⁻¹ₜ
It follows that:
(∂𝑇/∂𝑃)ₕ = −(∂𝐻/∂𝑃)ₜ(∂𝑇/∂𝐻)ₚ
Since:
(∂𝐻/∂𝑇)ₚ = 𝐶𝑝
Thus:
𝜇 = 1/𝐶𝑝[𝑇(∂𝑣ˉ/∂𝑇)ₚ−𝑣ˉ ]
Where 𝑣ˉ is the molar volume and 𝐶ₚ is the heat capacity at constant pressure. For real gases, this is further expressed as:
𝜇=𝑅𝑇²/𝑃𝐶ₚ(∂𝑍/∂𝑇)ₚ
This isenthalpic process, described in terms of pressure, temperature, and volume, demonstrates how the Joule-Thomson coefficient relates to the physical properties of the gas.
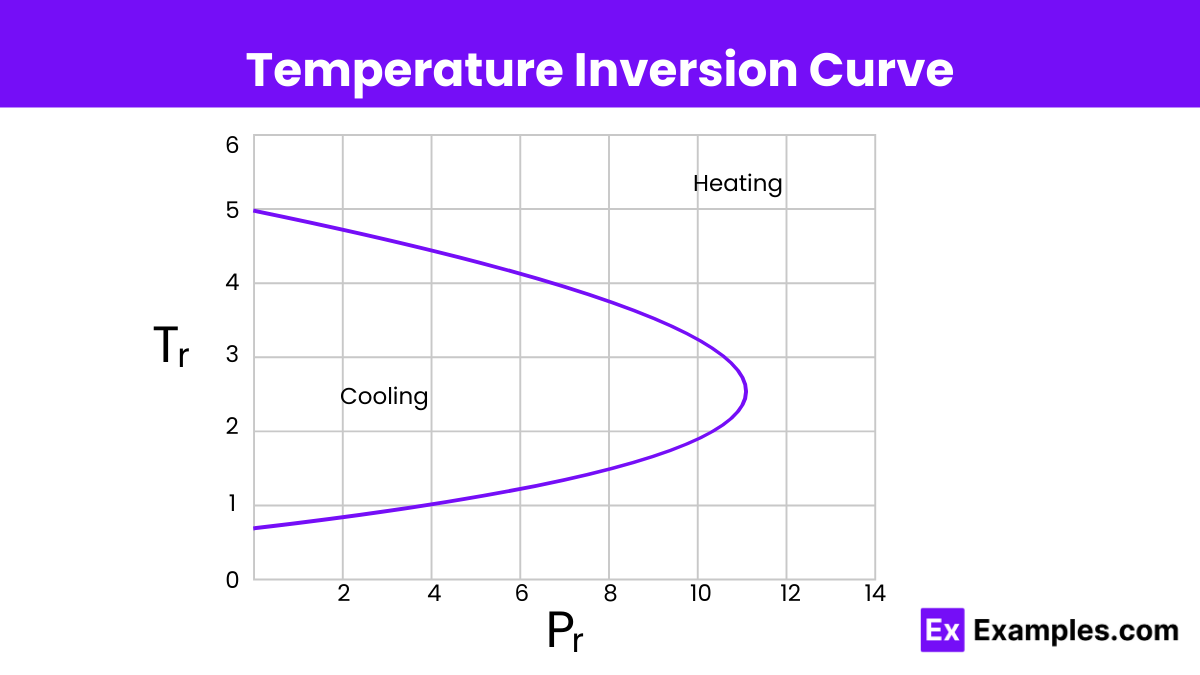
The inversion temperature is the temperature of a gas at which reducing the pressure does not change the temperature. Above this temperature, the gas heats up during expansion, while below this temperature, the gas cools down during expansion.
James Prescott Joule and William Thomson (Lord Kelvin) discovered the Joule-Thomson effect in the 1850s.
The effect occurs during gas expansion or compression through a porous plug or valve without heat exchange.
The temperature change depends on the initial temperature and the specific properties of the gas.
The Joule-Thomson coefficient measures the change in temperature per unit change in pressure during a throttling process.
No, gases cool or warm upon expansion depending on their initial temperature and Joule-Thomson coefficient.
Pressure differences drive the expansion or compression that causes the temperature change in the Joule-Thomson effect.
The effect is primarily observed in gases, as liquids have incompressible properties that prevent similar behavior.
The inversion temperature is the temperature at which a gas neither cools nor warms during expansion.
Gases like nitrogen, oxygen, and carbon dioxide exhibit significant cooling below their inversion temperatures.
The first law of thermodynamics, involving energy conservation, underlies the Joule-Thomson effect.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the Joule-Thomson effect?
A temperature change in a gas when it expands without doing work
A pressure change in a liquid when it is compressed
A temperature change in a solid when it is heated
A volume change in a gas when it is heated
Which of the following gases exhibits cooling during the Joule-Thomson expansion at room temperature?
Helium
Hydrogen
Nitrogen
Neon
What is the Joule-Thomson coefficient?
The change in temperature with respect to pressure at constant enthalpy
The change in pressure with respect to volume at constant temperature
The change in volume with respect to temperature at constant pressure
The change in enthalpy with respect to temperature at constant pressure
For which type of gas is the Joule-Thomson effect most pronounced?
Ideal gas
Real gas
Incompressible gas
Perfect gas
At what condition does the Joule-Thomson effect become zero for an ideal gas?
High temperature
Low temperature
High pressure
Any condition
Which property remains constant during the Joule-Thomson expansion?
Temperature
Pressure
Enthalpy
Volume
What happens to the temperature of a real gas with a positive Joule-Thomson coefficient during expansion?
It increases
It decreases
It remains constant
It oscillates
Which of the following parameters affects the Joule-Thomson coefficient?
Specific heat capacity
Thermal conductivity
Molecular interactions
Electric potential
What is the significance of the inversion temperature in the Joule-Thomson effect?
It is the temperature at which the gas liquefies
It is the temperature at which the Joule-Thomson coefficient changes sign
It is the temperature at which the gas becomes ideal
It is the temperature at which the gas solidifies
Which equation is used to determine the Joule-Thomson coefficient for a real gas?
Van der Waals equation
Ideal gas law
Clausius-Clapeyron equation
Bernoulli\'s equation
Before you leave, take our quick quiz to enhance your learning!

