Which of the following units is commonly used to measure heat in the United States?
Calorie
Joule
British Thermal Unit (BTU)
Watt

Heat is a form of energy that is transferred between systems or bodies with different temperatures. This fundamental concept is critical in the fields of thermodynamics, engineering, and environmental science.
Transitioning to more traditional measures, the Calorie (cal) is particularly prominent in nutritional science, where it describes the energy content in food, indicating how much heat energy the body can obtain from consuming it.
The SI unit of heat is the joule (J). Heat, a form of energy transfer, quantifies how much energy is exchanged between systems due to temperature differences. The joule, named after James Prescott Joule, measures this energy transfer effectively. It signifies the amount of energy used when a force of one newton is applied over a distance of one meter.
The British Thermal Unit (BTU) is a unit of heat used primarily in the United States. It measures the amount of heat required to raise the temperature of one pound of water by one degree Fahrenheit. The BTU is commonly employed in various applications including heating and cooling systems, cooking appliances, and energy consumption in industrial processes.
In the CGS (centimeter-gram-second) system of units, the unit of heat is the calorie (cal). One calorie is defined as the amount of heat required to raise the temperature of one gram of water by one degree Celsius at atmospheric pressure. This unit is particularly useful in chemistry and the food industry to describe the energy content of substances and reactions.
Temperature is a measure of the average kinetic energy of the particles in a substance. It is an indicator of how hot or cold a substance is and is typically measured in degrees Celsius (°C), Fahrenheit (°F), or Kelvin (K). Temperature provides a relative measure, allowing us to assess whether energy will transfer from one object to another. It does not, however, indicate the total energy present in a substance.
Heat refers to the transfer of thermal energy between systems or bodies that are at different temperatures. Heat is a form of energy in transit; it is not contained within an object but moves from one place to another. Heat can be measured in joules (J) or British Thermal Units (BTU) and is only evident when there is a temperature difference that causes energy to flow from a warmer area to a cooler one.
Internal Energy is the total energy stored within a system or substance. It encompasses all the kinetic and potential energies of the particles making up the system. This includes energies related to the motion of particles (kinetic) and energies due to the arrangement of particles or their interaction with each other (potential). Internal energy is a state function, which means it is dependent only on the current state of the system, not on how that state was achieved.
| Unit | Symbol |
|---|---|
| Joule | J |
| Kilowatt-hour | kWh |
| Calorie | cal |
| Kilocalorie | kcal |
| Electronvolt | eV |
| British Thermal Unit | BTU |
| Foot-pound | ft-lb |
The Joule is the SI unit of energy, named after James Prescott Joule. It measures the energy transferred when a force of one newton is exerted over a distance of one meter. It is universally used in science and engineering to quantify energy, work, or heat.
Known also as a food calorie, the kilocalorie is primarily used to express the energy content in foods, equivalent to 1000 smaller calories. It is important in dietary energy contexts to determine caloric intake.
The British Thermal Unit is predominantly used in the United States within heating and cooling systems. It defines the amount of energy needed to raise the temperature of one pound of water by one degree Fahrenheit.
A therm is a unit of heat commonly used in the energy industry, particularly in natural gas billing in the United States. It represents 100,000 BTUs, measuring large-scale energy consumption.
The watt-hour is a smaller unit of energy typically used in electricity measurements. It represents the energy produced or consumed by a power of one watt sustained for one hour.
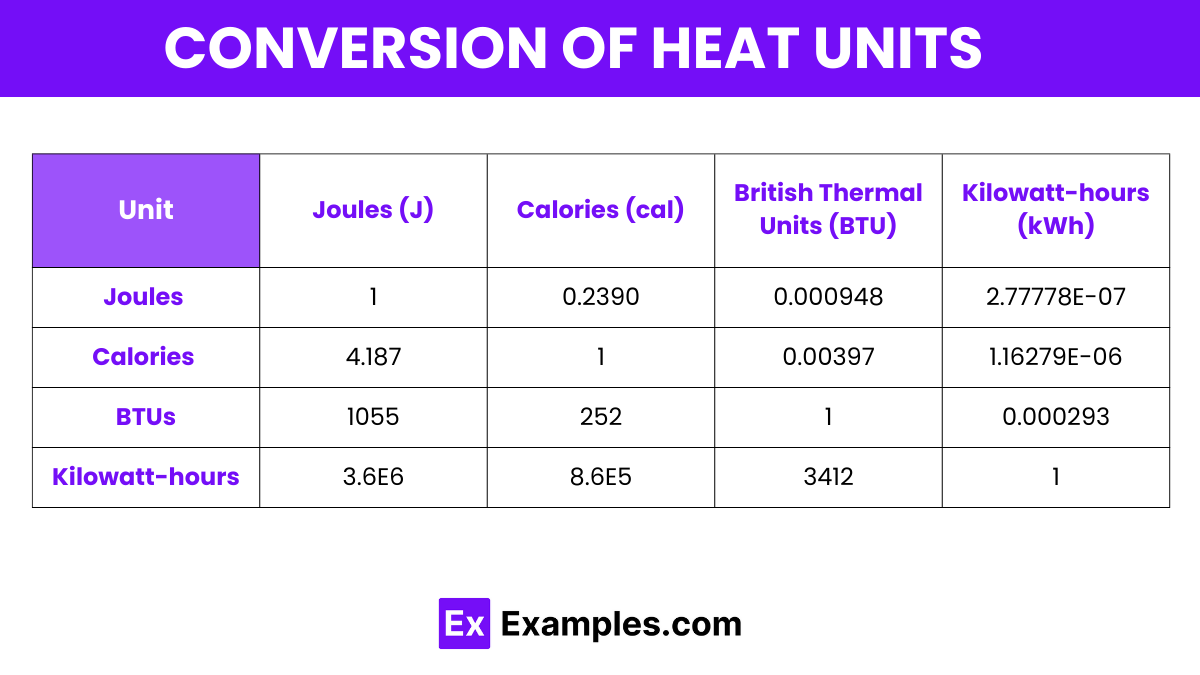
Here’s a conversion table for various units of heat, presenting how different heat units relate to each other, specifically Joules, Calories, BTUs, and Kilowatt-hours:
| Unit | Joules (J) | Calories (cal) | British Thermal Units (BTU) | Kilowatt-hours (kWh) |
|---|---|---|---|---|
| Joules (J) | 1 | 0.2390 | 0.000948 | 2.77778E-07 |
| Calories (cal) | 4.187 | 1 | 0.00397 | 1.16279E-06 |
| BTUs | 1055 | 252 | 1 | 0.000293 |
| Kilowatt-hours | 3.6E6 | 8.6E5 | 3412 | 1 |
100 J / 4.184 = 23.88 cal
500 J / 1055 ≈ 0.474 BTU
18000 J / 3.6 × 10^6 ≈ 0.005 kWh50 cal × 4.184 = 209.2 J100 cal × 0.00397 = 0.397 BTU1000 cal / 860,420 ≈ 0.00116 kWh2 BTU × 1055 = 2110 J5 BTU × 252 = 1260 cal10 BTU / 3412 ≈ 0.00293 kWh1 kWh × 3.6 × 10^6 = 3.6 × 10^6 J0.5 kWh × 860,420 = 430,210 cal0.1 kWh × 3412 = 341.2 BTUHeat is measured in joules (J) in the SI system, but calories (cal) and British Thermal Units (BTU) are also widely used.
The biggest common unit of heat is the kilocalorie (kcal), primarily used in dietary energy contexts, translating large amounts of heat energy.
The calorie, an older unit, was traditionally used to measure heat based on the energy needed to raise the temperature of water by one degree Celsius.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Which of the following units is commonly used to measure heat in the United States?
Calorie
Joule
British Thermal Unit (BTU)
Watt
How many joules are there in one calorie?
1.42 J
3.14 J
4.18 J
6.28 J
What is the definition of a calorie?
The amount of heat required to raise the temperature of 1 gram of water by 1 degree Celsius
The amount of heat required to raise the temperature of 1 kilogram of water by 1 degree Celsius
The amount of heat required to raise the temperature of 1 gram of water by 1 degree Fahrenheit
The amount of heat required to raise the temperature of 1 kilogram of water by 1 degree Fahrenheit
If a system absorbs 500 calories of heat, how many joules of heat has it absorbed?
2090 J
2500 J
4180 J
5000 J
Which unit is larger, a kilocalorie or a calorie?
Calorie
Kilocalorie
Both are the same
It depends on the context
What is the energy equivalent of 1 BTU in joules?
4.18 J
418 J
1054 J
2048 J
How many kilojoules are there in 1000 joules?
0.1 kJ
1 kJ
10 kJ
100 kJ
Which of the following is a unit of heat energy used in the food industry?
Calorie
BTU
Therm
Electronvolt
What is the heat capacity of water in calories per gram per degree Celsius?
0.5 cal/g°C
1 cal/g°C
2 cal/g°C
4 cal/g°C
How much heat is required to raise the temperature of 2 kg of water by 10°C? (Specific heat capacity of water = 4.18 J/g°C)
41.8 kJ
83.6 kJ
167.2 kJ
200 kJ
Before you leave, take our quick quiz to enhance your learning!

