Hemoglobin vs Myoglobin
Hemoglobin and myoglobin are essential proteins in the human body. They both bind and transport oxygen, but they have distinct roles and structures. Hemoglobin, found in red blood cells, transports oxygen from the lungs to tissues throughout the body. Myoglobin, located in muscle cells, stores oxygen and releases it during muscle contraction. Understanding the differences between hemoglobin and myoglobin is crucial for grasping how our bodies efficiently use oxygen to sustain life. This article will explore the key differences and similarities between these two vital proteins.
What is Hemoglobin?
Hemoglobin is a protein found in the red blood cells of most vertebrates, including humans. Its primary function is to transport oxygen from the lungs to the body’s tissues and to help return carbon dioxide from the tissues back to the lungs to be exhaled. Hemoglobin is what gives red blood cells their color and is crucial for carrying oxygen throughout the body to maintain cellular function.
Structure of Hemoglobin
Hemoglobin is composed of four protein subunits, each of which can bind to one molecule of oxygen. The protein is made up of two alpha (α) and two beta (β) chains in adults, each with a heme group that contains an iron atom at its center. This iron atom is where oxygen binds; each hemoglobin molecule can carry up to four oxygen molecules.
Function of Hemoglobin
- Oxygen Binding: Oxygen molecules bind to the iron in the heme groups as blood flows through the lungs.
- Oxygen Transport: Once bound, oxygen is carried through the bloodstream to tissues where it is needed.
- Carbon Dioxide Transport: After delivering oxygen, hemoglobin picks up carbon dioxide, a waste product of metabolism, and transports it back to the lungs, where it is expelled from the body.
Clinical Significance
- Anemia: Low hemoglobin levels may indicate anemia, a condition where the body doesn’t have enough healthy red blood cells.
- Polycythemia: High hemoglobin levels might be seen in polycythemia, a condition where the body makes too many red blood cells.
- Hemoglobinopathies: Disorders like sickle cell anemia and thalassemia involve structural abnormalities in the hemoglobin molecule, which can lead to various health problems including severe anemia and complications related to impaired oxygen transport.
Measurement and Importance
Hemoglobin levels are commonly measured using a complete blood count (CBC) test, which is part of routine health check-ups. Monitoring these levels is crucial for diagnosing and managing disorders related to red blood cells. Optimal hemoglobin levels vary by age, sex, and overall health, but generally, normal ranges are:
- Men: 13.8 to 17.2 grams per deciliter
- Women: 12.1 to 15.1 grams per deciliter
Maintaining appropriate hemoglobin levels is essential for ensuring that the body’s tissues have enough oxygen to function effectively and for preventing complications associated with both low and high hemoglobin levels.
What is Myoglobin?
Myoglobin is a protein found primarily in the muscle tissues of vertebrates, including humans. It serves as an oxygen-storage unit, providing oxygen to the muscle cells during times of high demand, such as during vigorous exercise. Myoglobin helps in the efficient use of oxygen by muscle cells and plays a crucial role in enabling muscle tissue to maintain high levels of metabolic activity.
Structure of Myoglobin
Myoglobin is similar to a single subunit of hemoglobin, composed of a single polypeptide chain and a heme group. The heme group contains an iron atom that binds to oxygen. The structure of myoglobin allows it to bind oxygen more tightly than hemoglobin, making it an effective oxygen reservoir for muscle cells.
Function of Myoglobin
- Oxygen Storage: Myoglobin stores oxygen when it is abundant, particularly when muscles are at rest.
- Oxygen Release: During intense muscle activity, when oxygen levels are low, myoglobin releases its stored oxygen to sustain energy production in muscle cells.
Clinical Significance
- Muscle Injury Indicator: High levels of myoglobin in the blood can indicate muscle injury, including severe conditions like rhabdomyolysis, where muscle tissue breaks down rapidly.
- Diagnostic Tool: Testing for myoglobin levels in the blood can help diagnose acute myocardial infarction (heart attack) and other muscle-related injuries, though other markers like troponin are now more commonly used due to their higher specificity.
Measurement and Importance
Myoglobin can be measured in the blood, where increases are often associated with muscle damage. It is also measured in urine to assess for rhabdomyolysis, especially if the urine appears dark, which can be a sign of myoglobinuria (presence of myoglobin in urine). Monitoring myoglobin levels can be important in the clinical setting to assess the extent of muscle damage and to guide treatment strategies.
Differences between Hemoglobin and Myoglobin
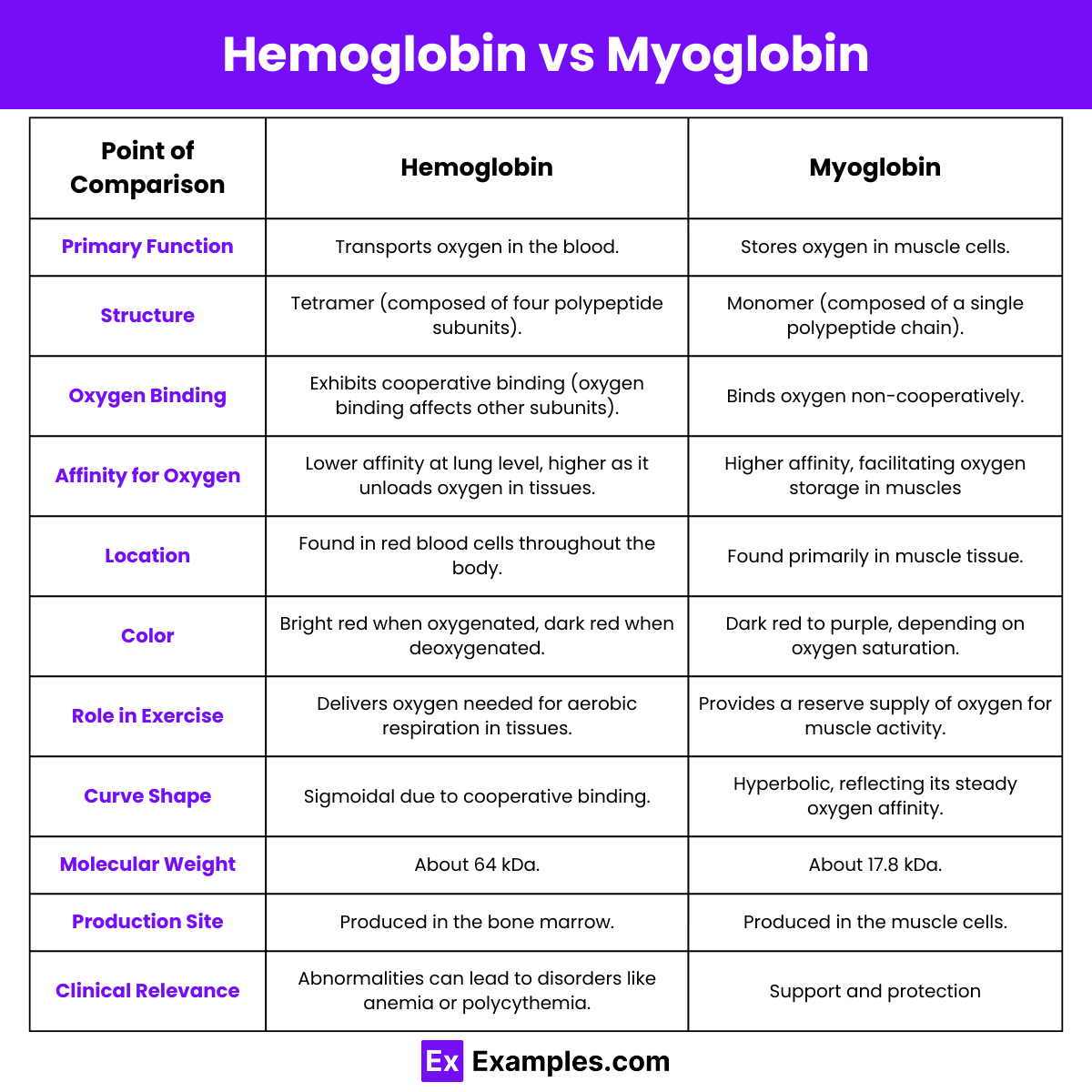
| Feature | Hemoglobin | Myoglobin |
|---|---|---|
| Function | Transports oxygen in the blood. | Stores oxygen in muscle tissues. |
| Structure | Tetramer (four subunits: 2 alpha and 2 beta). | Monomer (single subunit). |
| Binding Sites | Four oxygen-binding sites. | One oxygen-binding site. |
| Oxygen Affinity | Lower affinity, variable (cooperative binding). | Higher affinity, constant. |
| Cooperativity | Exhibits cooperativity. | Does not exhibit cooperativity. |
| Location | Found in red blood cells. | Found in muscle cells. |
| Binding Curve | Sigmoidal due to cooperativity. | Hyperbolic, indicating no cooperativity. |
| Role in Oxygen Transport | Carries oxygen from lungs to tissues. | Facilitates oxygen use within muscles. |
| Molecular Weight | About 64,500 daltons. | About 17,800 daltons. |
| Primary Function in Tissues | Delivers oxygen based on tissue needs. | Provides a steady supply of oxygen. |
| Color | Bright red when oxygenated, darker without oxygen. | Purplish red. |
| Response to pH | Bohr effect: Affinity decreases with lower pH. | Less sensitive to pH changes. |
| Heme Groups | Contains four heme groups. | Contains one heme group. |
| Production Site | Produced in the bone marrow. | Produced in muscle cells. |
| Presence in Animals | Found in all vertebrates. | Primarily found in vertebrates. |
Similarities Between Hemoglobin and Myoglobin
Structure
Both hemoglobin and myoglobin are heme-containing proteins that bind oxygen. They share a similar structural motif in their heme group, which is where oxygen binding occurs. The heme group consists of an iron atom held in a porphyrin ring, crucial for oxygen binding.
Function in Oxygen Binding
Hemoglobin and myoglobin both function in oxygen transport and storage. Myoglobin, found primarily in muscle tissue, stores oxygen and releases it during muscle contraction when oxygen levels are low. Hemoglobin, found in red blood cells, transports oxygen from the lungs to other parts of the body and facilitates carbon dioxide removal.
Amino Acid Composition
Both proteins are composed of amino acids that create a three-dimensional structure capable of binding oxygen. While their overall sequences and structures differ, the presence of specific amino acids at the heme binding site is conserved, allowing both to perform their oxygen-binding function effectively.
Evolutionary Relationship
Hemoglobin and myoglobin are evolutionarily related and belong to the same protein family. This relationship is evident in their structural similarities and their mechanism of oxygen binding. Both proteins have evolved to optimize oxygen binding and release in response to different physiological demands and environments.
How do hemoglobin and myoglobin differ in structure?
Hemoglobin has four protein subunits, whereas myoglobin consists of a single polypeptide chain, making it simpler in structure.
What is the primary function of hemoglobin?
Hemoglobin’s primary function is to transport oxygen from the lungs to the body’s tissues.
What is the primary function of myoglobin?
Myoglobin stores oxygen in muscle tissues, releasing it during intense physical activity.
How do their oxygen-binding capacities differ?
Hemoglobin releases oxygen based on tissue demand, showing cooperative binding, while myoglobin has a higher affinity for oxygen and releases it at lower concentrations.
Where is hemoglobin found in the body?
Hemoglobin is primarily found in the red blood cells circulating throughout the body.
Where is myoglobin found in the body?
Myoglobin is found in the heart and skeletal muscles.
How do hemoglobin and myoglobin contribute to muscle color?
Myoglobin gives muscles a reddish color, more pronounced in darker, oxygen-rich muscles.
Can hemoglobin and myoglobin indicate health issues?
Yes, abnormalities in hemoglobin and myoglobin levels can indicate respiratory, muscular, and circulatory disorders.
Why is understanding hemoglobin and myoglobin important in medicine?
Understanding these molecules helps diagnose and treat conditions like anemia, muscle damage, and other oxygen-related issues.