Aluminum
Aluminum, a versatile and widely used metal, plays a significant role in various industries. Its unique properties, such as lightweight and corrosion resistance, make it a favorite among manufacturers and educators. This article offers an in-depth look at Aluminum, providing teachers with examples and practical applications to enhance their teaching methods. Whether it’s discussing its role in everyday objects or exploring its scientific characteristics, our guide is an essential resource for educators looking to enrich their curriculum with real-world examples of Aluminum.
What is Aluminum?
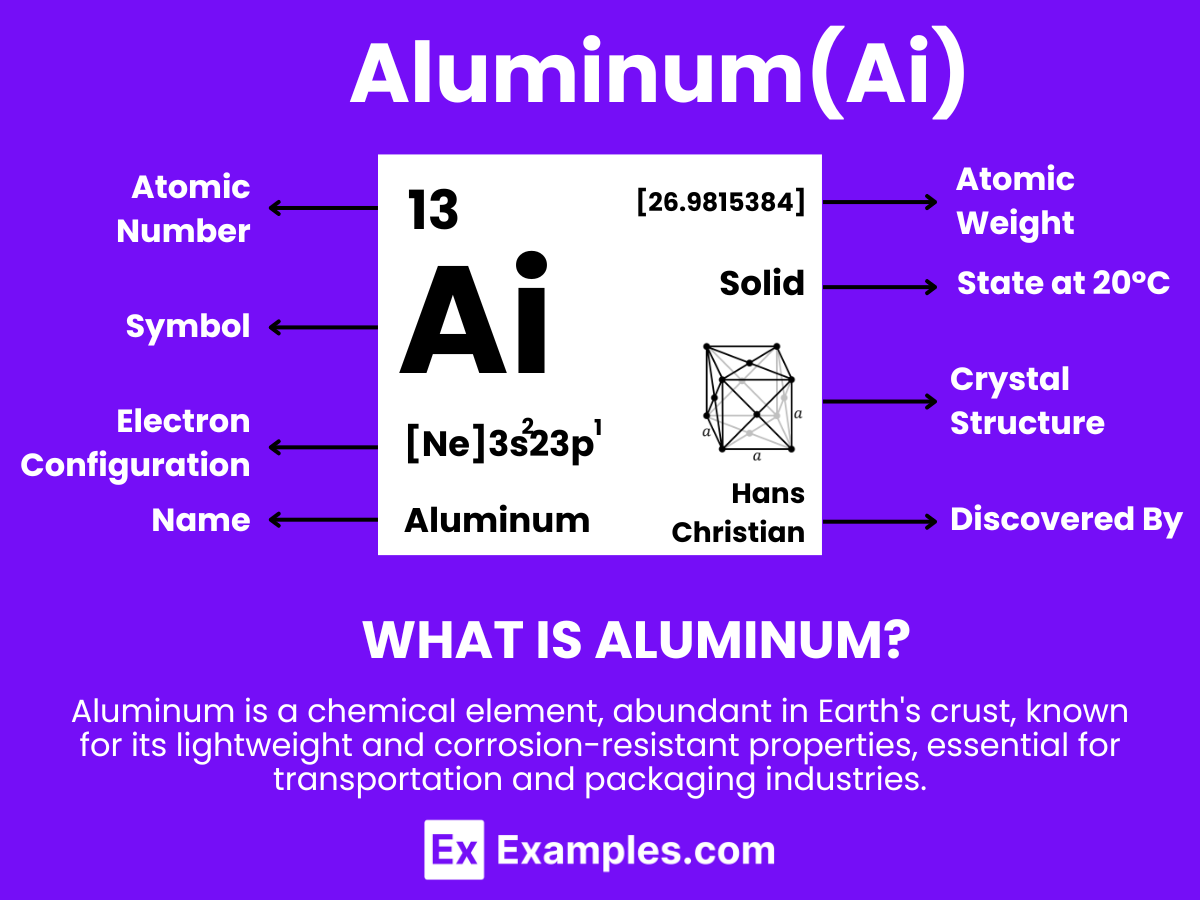
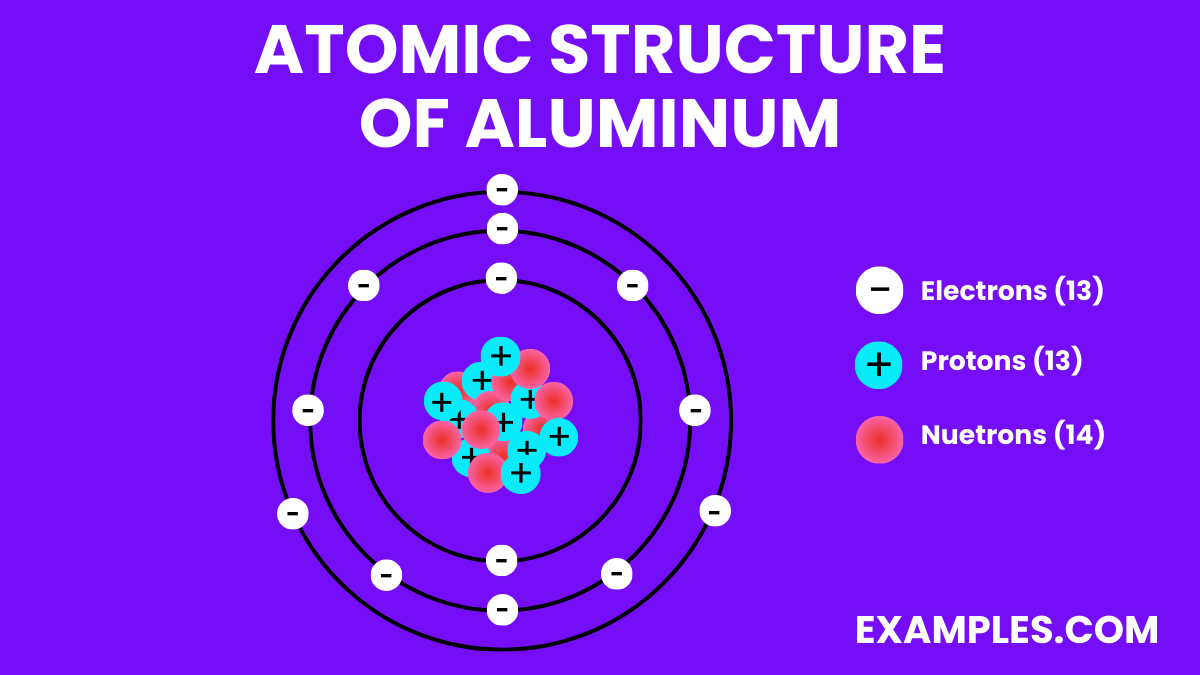
Aluminum is a chemical element with the symbol Al and atomic number 13. It is a silvery-white, soft, non-magnetic metal. Being the most abundant metal in the Earth’s crust, it’s used extensively in a wide range of applications. Aluminum is known for its lightweight, durability, and resistance to corrosion, which makes it ideal for use in industries such as aerospace, transportation, construction, and packaging. In the classroom, Aluminum can serve as an excellent example when teaching about elements, material properties, and their applications in modern technology and everyday life.
Aluminum Formula
- Formula: Al
- Composition: A single aluminium atom.
- Bond Type: Aluminium forms metallic bonds in its bulk form and covalent bonds in compounds, due to its three valence electrons.
- Molecular Structure: Silvery-white, reflective, and has a face-centered cubic crystal structure in its most stable form.
- Electron Configuration: 13 electrons, with the configuration 1s² 2s² 2p⁶ 3s² 3p¹.
- Significance: Widely used in aerospace, transportation, and packaging due to its light weight and resistance to corrosion.
- Role in Chemistry: Essential in forming various compounds and alloys; used in materials science and physical chemistry.
Structure of Aluminum Gas
Aluminum gas does not occur under normal environmental conditions due to aluminum’s high melting and boiling points. It transitions into a gaseous state only at extremely high temperatures (above 2,470 degrees Celsius or 4,478 degrees Fahrenheit). In this state, aluminum exists as individual atoms rather than forming molecules like diatomic hydrogen (H₂). Here’s a simplified explanation of the structure of gaseous aluminum:
Atomic Level: Each aluminum atom consists of 13 protons in its nucleus surrounded by 13 electrons. Unlike hydrogen, which achieves stability through sharing electrons in a covalent bond, individual aluminum atoms in the gas phase do not pair up or form bonds with each other.
Gaseous Formation: Upon reaching its boiling point, aluminum atoms escape the solid or liquid phase as individual entities. In this gaseous state, the atoms are isolated and free-moving, without any direct bonding to other aluminum atoms. This lack of molecular formation is a notable difference from the diatomic nature of hydrogen gas.
The gaseous state of aluminum is characterized by these free-moving, unbonded atoms that exist individually rather than as part of a molecule. This state is achieved only under conditions of extreme heat, which provides enough energy to overcome the strong metallic bonds that normally hold the aluminum atoms together in a solid or liquid form.
Properties of Aluminum
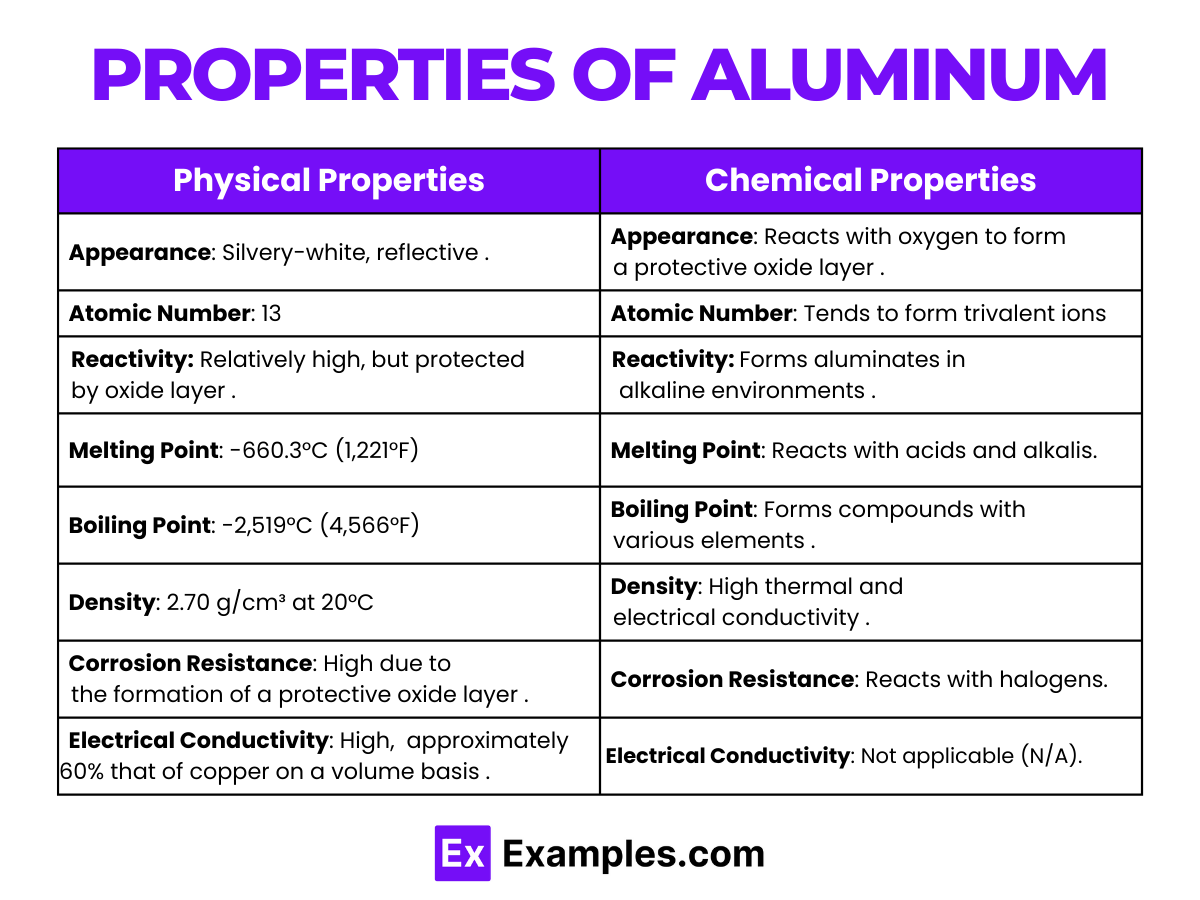
Physical Properties of Aluminum
| Property | Description |
|---|---|
| Appearance | Silvery-white, reflective, and lightweight metal |
| Melting Point | 660.3 °C (1,221 °F) |
| Boiling Point | 2,470 °C (4,478 °F) |
| Density | 2.70 g/cm³ at 20 °C |
| Malleability | Extremely malleable, can be rolled into thin sheets |
| Conductivity | Excellent conductor of electricity and heat |
| Ductility | Highly ductile, allowing it to be drawn into thin wires |
| Strength-to-Weight Ratio | High, making it ideal for aerospace and transportation applications |
Chemical Properties of Aluminum
- Reaction with Air:
- Aluminum reacts with oxygen in the air to form a thin layer of aluminum oxide, Al₂O₃, which protects it from further oxidation.
- Equation:
- Reaction with Acids:
- Aluminum reacts with dilute hydrochloric acid to form aluminum chloride (AlCl₃) and hydrogen gas.
- Equation:
- Reaction with Bases:
- It reacts with bases like sodium hydroxide to produce sodium aluminate and hydrogen gas.
- Equation:
- Resistance to Corrosion:
- Due to the formation of the oxide layer, aluminum is highly resistant to corrosion.
- Alloy Formation:
- Aluminum forms alloys with other metals like copper, magnesium, manganese, silicon, and zinc, enhancing its mechanical properties.
- Amphoteric Nature:
- Aluminium oxide, Al₂O₃, exhibits amphoteric behavior, meaning it can react with both acids and bases.
- Electropositivity:
- As a relatively electropositive metal, aluminum tends to lose electrons in chemical reactions, commonly forming the ion.
- Thermal Stability:
- Aluminum compounds generally have high melting points and are thermally stable.
Thermodynamic Properties of Aluminum
| Property | Description / Value |
|---|---|
| Melting Point | 660.3°C (1220.5°F) |
| Boiling Point | 2519°C (4566°F) |
| Thermal Conductivity | 235 W/(m·K) |
| Specific Heat | 0.897 J/(g·K) at 25°C |
| Heat of Vaporization | 294 kJ/mol |
| Heat of Fusion | 10.7 kJ/mol |
Material Properties of Aluminum
| Property | Description / Value |
|---|---|
| Phase at STP | Solid |
| Density | 2.70 g/cm³ at 20°C |
| Young’s Modulus | 70 GPa |
| Tensile Strength | 90 to 690 MPa (depending on alloy) |
| Mohs Hardness | 2.75 |
| Elastic Modulus | 69 GPa |
Electromagnetic Properties of Aluminum
| Property | Description / Value |
|---|---|
| Magnetic Susceptibility | Diamagnetic |
| Electrical Conductivity | 37.7 MS/m at 20°C |
| Reflectivity | About 80% (visible light) |
Nuclear Properties of Aluminum
| Property | Description / Value |
|---|---|
| Atomic Number | 13 |
| Atomic Mass | 26.9815385 u |
| Neutron Cross Section | 0.231 barns (for ^27Al) |
| Isotopes | ^27Al (100% natural abundance) |
| Radioactivity | Aluminum is not radioactive, but it has artificial radioactive isotopes like ^26Al with a half-life of 717,000 years |
Chemical Compounds of Aluminum
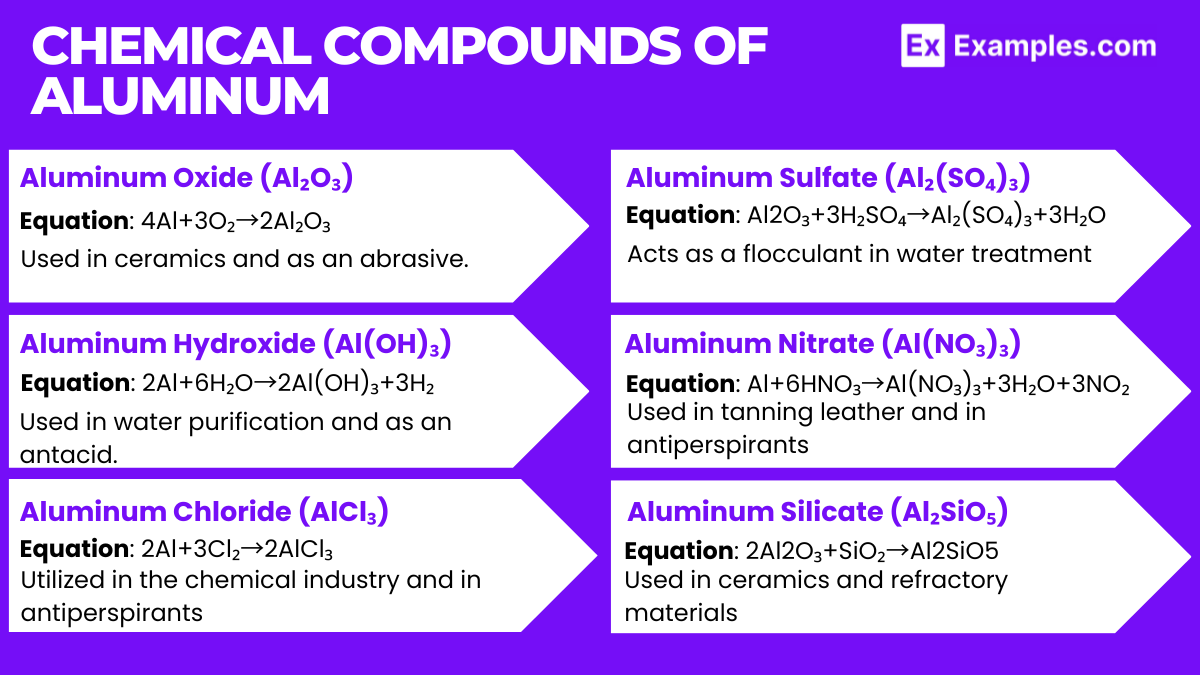
- Aluminum Oxide (Al₂O₃):
- This compound is commonly known as alumina.
- Equation:
- Used in ceramics and as an abrasive.
- Aluminum Hydroxide (Al(OH)₃):
- Formed when aluminum reacts with water.
- Equation:
- Used in water purification and as an antacid.
- Aluminum Chloride (AlCl₃):
- Produced from the reaction of aluminum and chlorine.
- Equation:
- Utilized in the chemical industry and in antiperspirants.
- Aluminum Sulfate (Al₂(SO₄)₃):
- Commonly used in water purification and papermaking.
- Equation:
- Acts as a flocculant in water treatment.
- Aluminum Nitrate (Al(NO₃)₃):
- Formed from the reaction of aluminum with nitric acid.
- Equation:
- Used in tanning leather and in antiperspirants.
- Aluminum Silicate (Al₂SiO₅):
- Exists in various forms including kyanite, andalusite, and sillimanite.
- Equation: 2Al2O+SiO→Al2SiO5.
- Used in ceramics and refractory materials.
Isotopes of Aluminum
| Isotope | Natural Abundance | Half-Life | Application/Use |
|---|---|---|---|
| Al-26 | Trace amounts | 720,000 years | Used in radiodating, particularly in meteorite studies. |
| Al-27 | 100% | Stable | The only stable isotope, used in NMR spectroscopy. |
| Al-28 | Synthetic | 2.24 minutes | Produced in particle accelerators, used in research. |
| Al-29 | Synthetic | 6.56 minutes | Used in scientific experiments, particularly in nuclear physics research. |
| Al-30 | Synthetic | 3.6 seconds | Studied in nuclear physics and astrophysics research. |
| Al-31 | Synthetic | 644 milliseconds | Mainly of interest for scientific research. |
Uses of Aluminum
Transportation
Aluminum is extensively used in the transportation industry due to its high strength-to-weight ratio. This property makes vehicles lighter, which improves fuel efficiency. It’s commonly used in the manufacture of car bodies, airplane frames, and railway carriages.
Packaging
One of the most familiar uses of aluminum is in packaging. Its ability to form a barrier against moisture, light, and oxygen makes it ideal for preserving food and beverages. Aluminum foil and cans are commonplace in the food industry.
Construction
Aluminum’s durability and resistance to corrosion make it an excellent material for building and construction. It’s used in window frames, doors, roofing, and siding. Aluminum’s reflective surface also helps in insulation.
Electrical
Due to its excellent electrical conductivity, aluminum is widely used in electrical transmission lines. It’s lighter and less expensive than copper, making it the preferred choice for wiring in residential and commercial buildings.
Consumer Goods
Aluminum is used in a wide range of consumer goods, including electronics like smartphones and laptops, due to its lightweight and heat-conductive properties. It’s also found in cooking utensils, furniture, and decorative items.
Aerospace
The aerospace industry relies heavily on aluminum for constructing aircraft. Its light weight, strength, and resistance to corrosion are crucial for the performance and safety of airplanes and spacecraft.
Commercial Production of Aluminum
Aluminum, known for its versatility and lightweight, is a widely used metal with a complex production process. The commercial production of aluminum primarily involves two key stages: the Bayer process and the Hall-Héroult process.
Bayer Process
- Bauxite Mining: It begins with the mining of bauxite, the primary ore of aluminum. This ore typically contains 30-60% aluminum oxide (Al2O3).
- Crushing and Grinding: The bauxite is then crushed and ground into a fine powder.
- Digestion: The powder is mixed with a hot solution of sodium hydroxide, which digests the aluminum oxide, forming sodium aluminate.
- Settling and Clarification: Impurities settle to the bottom, and the clear solution containing dissolved sodium aluminate is decanted off.
- Precipitation: The solution is cooled, and aluminum hydroxide precipitates out.
- Calcination: The aluminum hydroxide is then heated in rotary kilns or fluidized bed calciners to remove water, producing alumina (Al2O3).
Hall-Héroult Process
- Electrolysis: The alumina is dissolved in molten cryolite, and electrical current is passed through this solution.
- Reduction to Aluminum: This causes the alumina to break down into aluminum and oxygen. The aluminum collects at the bottom of the cell.
- Casting: The molten aluminum is tapped from the cell, cooled, and formed into ingots, sheets, or other shapes.
Through these processes, aluminum is produced in various forms, including sheets, foils, powders, and more, for use in a multitude of applications like packaging, transportation, construction, and electronics.
Health Effects of Aluminum
While aluminum is a common element in the Earth’s crust and widely used in various products, its health effects have been a subject of study and discussion.
Positive Aspects
- Low Toxicity: Generally, aluminum has a low toxicity level for humans.
- Limited Absorption: Most aluminum intake through food, water, and medicine is not absorbed by the body and is excreted via the kidneys.
Concerns
- Neurological Effects: There have been concerns about the potential link between aluminum exposure and neurological disorders, including Alzheimer’s disease. However, no definitive evidence has been established.
- Exposure in Workplaces: Workers in industries where aluminum dust or fumes are present may experience respiratory issues or dermatitis.
- Overexposure: High levels of aluminum exposure can lead to conditions like bone diseases or impaired kidney function.
It is crucial to understand that the everyday use of aluminum in cookware, cans, and foil is generally considered safe. Health risks are primarily associated with excessive exposure.
Environmental Effects of Aluminum
The production and use of aluminum have several environmental impacts, which are increasingly being addressed through various measures.
Impacts
- Mining Impact: Bauxite mining for aluminum production can lead to deforestation, soil erosion, and loss of biodiversity.
- Energy Consumption: The Hall-Héroult process is energy-intensive, contributing to significant greenhouse gas emissions.
- Waste Management: Red mud, a byproduct of the Bayer process, needs careful handling due to its caustic nature.
Mitigation Efforts
- Recycling: Aluminum is highly recyclable, and recycling it consumes only 5% of the energy used to produce new aluminum.
- Improving Efficiency: Technological advancements aim to reduce energy consumption in aluminum production.
- Rehabilitation of Mining Sites: Efforts are made to restore the ecological balance after bauxite mining.
What is the Difference Between Aluminum and Aluminium?
“Aluminum” and “Aluminium” refer to the same element; “Aluminum” is used in American English, while “Aluminium” is used in British English.
What’s in Aluminum?
Aluminum is a pure element with the symbol Al and atomic number 13. It’s known for being lightweight and silvery-white in color.
Is Aluminum in the Periodic Table?
Yes, aluminum is in the periodic table, classified as a post-transition metal, located in Group 13.
Is Aluminum a Rare Element?
No, aluminum is not rare. It’s the most abundant metal in the Earth’s crust and the third most abundant element overall.
What Type of Metal is Aluminum?
Aluminum is a lightweight, ductile, and malleable metal, classified as a post-transition metal, known for its corrosion resistance.
aluminum stands out as a remarkably versatile and essential metal in modern society. Its unique properties, including lightness, strength, and resistance to corrosion, make it indispensable across various industries—from transportation and construction to packaging and electronics. The continuous innovation in aluminum production and recycling further underscores its role in sustainable development and technological advancement.