What is the chemical symbol for Americium?
Am
Ar
Ac
At
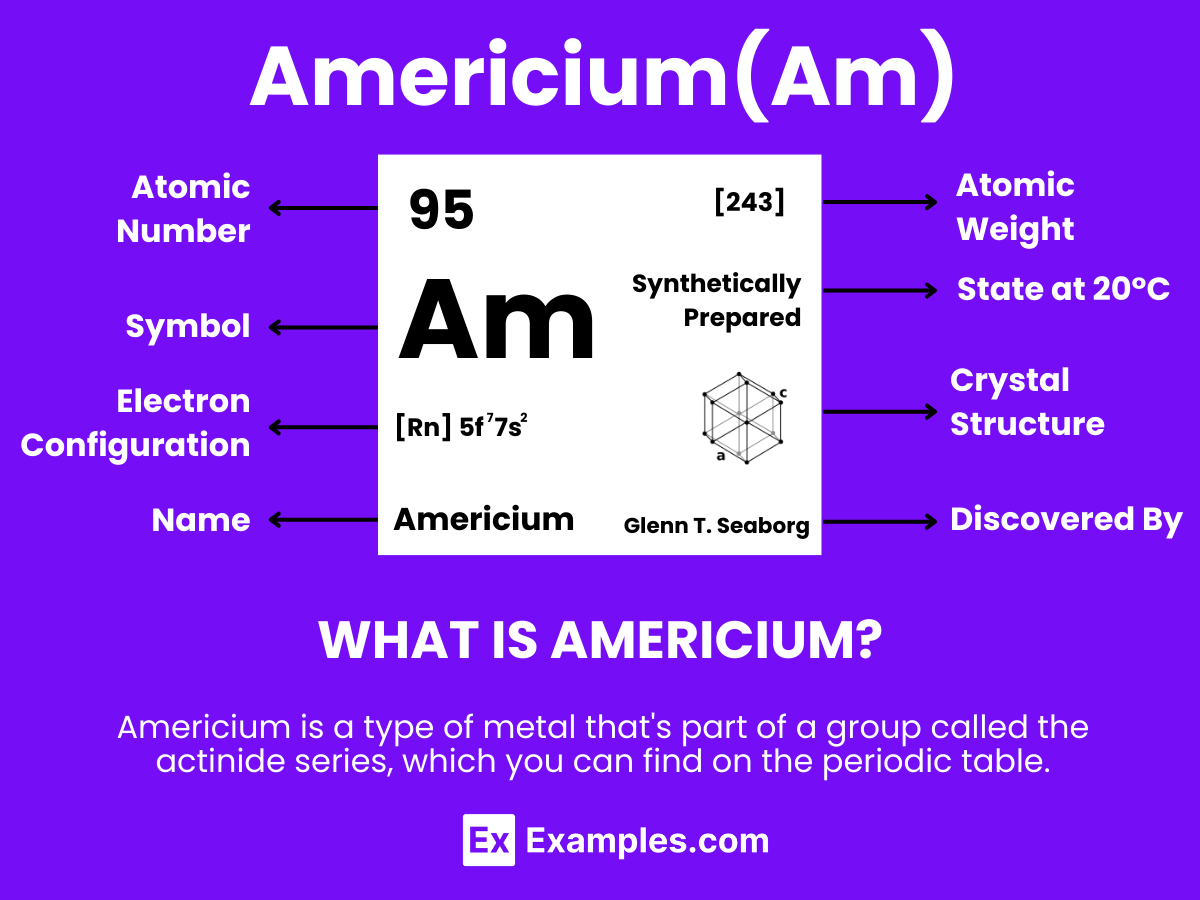
Explore the fascinating element of Americium, a cornerstone in the field of nuclear science and a critical component in smoke detectors. As a member of the actinide series, Americium plays a pivotal role in research, offering insights into nuclear reactions and properties. This complete guide delves into Americium’s discovery, its unique physical and chemical properties, and its diverse applications, from household safety to space exploration. Through practical examples, we illuminate the significant impact of Americium on technology, safety, and scientific advancement, highlighting its importance in our daily lives and beyond.
Americium is a silvery-white synthetic element that stands out for its unique properties and wide array of applications. With the atomic number 95, Americium is known for its radioactivity and ability to generate a significant amount of heat, making it particularly useful in specialized environments. This element does not occur naturally but is produced in nuclear reactors through the bombardment of plutonium. Americium is extensively utilized in various fields, especially in smoke detectors, where it serves as a source of ionizing radiation. .
| Actinium | Berkelium |
| Thorium | Californium |
| Protactinium | Einsteinium |
| Uranium | Curium |
| Neptunium | Mendelevium |
| Plutonium | Nobelium |
| Fermium | Lawrencium |
1. Fundamental Characteristics
2. Nuclear Composition
3. Electron Configuration
4. Radioactivity
5. Oxidation States
6. Applications and Uses
| Property | Value |
|---|---|
| Appearance | Silvery-white, glowing with an eerie blue light in the dark due to its radioactivity |
| Atomic Number | 95 |
| Atomic Weight | 243 |
| Density (at room temperature) | 13.69 g/cm³ |
| Melting Point | 1176 °C (2149 °F) |
| Boiling Point | 2607 °C (4725 °F) |
| State at Room Temperature | Solid |
| Electronegativity (Pauling scale) | 1.3 |
| Crystal Structure | Hexagonal |
| Heat of Fusion | 14.39 kJ/mol |
| Heat of Vaporization | 324 kJ/mol |
| Specific Heat Capacity | 0.181 J/(g·K) |
| Electrical Conductivity | Moderate, metallic |
| Thermal Conductivity | 10 W/(m·K) |
Americium, a synthetic element with the symbol Am and atomic number 95, is a member of the actinide series in the periodic table. It exhibits fascinating chemical properties, which are essential for various applications, including smoke detectors and in neutron sources.
Americium is a radioactive element that demonstrates a range of oxidation states, with +3 being the most stable and common. However, it can also exhibit other oxidation states, including +2, +4, +5, and +6 under specific conditions. This variability in oxidation states underlines Americium’s versatility in chemical reactions.
| Property | Value |
|---|---|
| Melting Point | 1176°C |
| Boiling Point | 2607°C |
| Density | 13.67 g/cm³ (at room temperature) |
| Heat of Fusion | 14.39 kJ/mol |
| Heat of Vaporization | 238.5 kJ/mol |
| Specific Heat Capacity | 62.7 J/mol·K |
| Property | Value |
|---|---|
| Phase at Room Temperature | Solid |
| Color | Silvery-white |
| Crystal Structure | Face-centered cubic (fcc) |
| Hardness | Relatively soft for a metal |
| Malleability | Ductile |
| Thermal Conductivity | 10 W/(m·K) |
| Property | Value |
|---|---|
| Electrical Conductivity | Low, typical for actinides |
| Magnetic Ordering | Paramagnetic at room temperature |
| Magnetic Susceptibility | Positive |
| Property | Value |
|---|---|
| Primary Isotopes | Americium-241 and Americium-243 |
| Radioactivity | Yes |
| Half-life of Am-241 | 432.2 years |
| Half-life of Am-243 | 7,370 years |
| Neutron Cross Section | High, particularly for Am-241 |
| Decay Modes | Alpha decay, with some isotopes undergoing spontaneous fission |
The preparation of Americium, a synthetic element with the symbol Am and atomic number 95, involves intricate nuclear reactions and processes. As an element not found naturally on Earth, Americium is produced in nuclear reactors through a series of neutron capture reactions and subsequent decay processes. Here’s an overview of how Americium is prepared:
| Isotope | Mass Number | Half-life | Decay Mode |
|---|---|---|---|
| Am-241 | 241 | 432.2 years | Alpha decay to Np-237 |
| Am-242m | 242 | 141 years | Beta decay to Cm-242 |
| Am-243 | 243 | 7,370 years | Alpha decay to Np-239 |
| Am-244 | 244 | 10.1 hours | Beta decay to Cm-244 |
| Am-242 | 242 | 16.02 hours | Beta decay to Cm-242 / Alpha decay to Pu-238 |
Americium, with the atomic symbol Am and atomic number 95, is a synthetic element that does not occur naturally on Earth. It is primarily produced in nuclear reactors through a series of neutron capture reactions involving lighter elements. The production process of Americium is both complex and fascinating, highlighting the intersection of nuclear physics and chemistry.
The most common method for producing Americium involves irradiating Plutonium (Pu) with neutrons in a nuclear reactor. Plutonium-239, a major component of spent nuclear fuel, serves as the starting material for Americium production.
After its formation, Americium is chemically separated from Plutonium and other fission products through a series of chemical processes. These may include solvent extraction, ion exchange, or precipitation techniques, tailored to isolate and purify Americium compounds effectively.
Americium’s unique properties, particularly its radioactivity and ability to emit alpha particles, make it suitable for a variety of applications. While handling Americium requires strict safety protocols due to its radiotoxicity, its uses in industry and research are significant.
Smoke Detectors: One of the most common uses of Americium is in smoke detectors. Americium-241, in the form of Americium dioxide (AmO2), is used as a source of alpha particles. These particles ionize air molecules, creating a current that can be disrupted by smoke particles, triggering the alarm. This application utilizes the Americium-241 isotope due to its relatively long half-life and alpha emission properties.
Neutron Sources: Americium-241 is also combined with Beryllium (Be) to form neutron sources. When Americium’s alpha particles hit Beryllium atoms, neutrons are emitted. These neutron sources are valuable in a range of applications, from oil well logging to neutron radiography and research.
Research and Medicine: In research, Americium’s isotopes are used in studies of the actinide series and in synthesizing new elements. Its alpha radiation is utilized in radiography and in certain types of radiation therapy for cancer treatment, though these applications are more limited compared to its use in smoke detectors and neutron sources.
Americium, a synthetic element produced in nuclear reactors, showcases remarkable chemical properties and versatile applications, from smoke detectors to neutron sources. Its unique capabilities underscore the importance of nuclear chemistry in advancing technology and safety. Understanding Americium’s production, properties, and uses highlights the integral role of synthetic elements in contemporary science and industry.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical symbol for Americium?
Am
Ar
Ac
At
Americium was named after which country?
Germany
United States
France
Russia
Which type of radiation is primarily emitted by Americium-241?
Alpha radiation
Beta radiation
Gamma radiation
Neutron radiation
In which year was Americium first discovered?
1944
1950
1939
1965
What is one common use of Americium-241 in household items?
Smoke detectors
Light bulbs
Refrigerators
Radios
Americium is classified as which type of element?
Alkali metal
Noble gas
Actinide
Halogen
What is the atomic number of Americium?
92
95
98
99
Americium can be used to produce which type of radiation in scientific experiments?
X-rays
Ultraviolet light
Infrared light
Microwaves
Which element is commonly used as a source of neutrons in scientific research, and is similar in its properties to Americium?
Plutonium
Uranium
Thorium
Radon
What is the primary challenge in handling Americium-241 safely?
Its high reactivity with water
Its extreme heat production
Its radioactive decay
Its strong magnetic properties
Before you leave, take our quick quiz to enhance your learning!

