What is the chemical symbol for Bismuth?
Bi
Bs
Bm
Bt
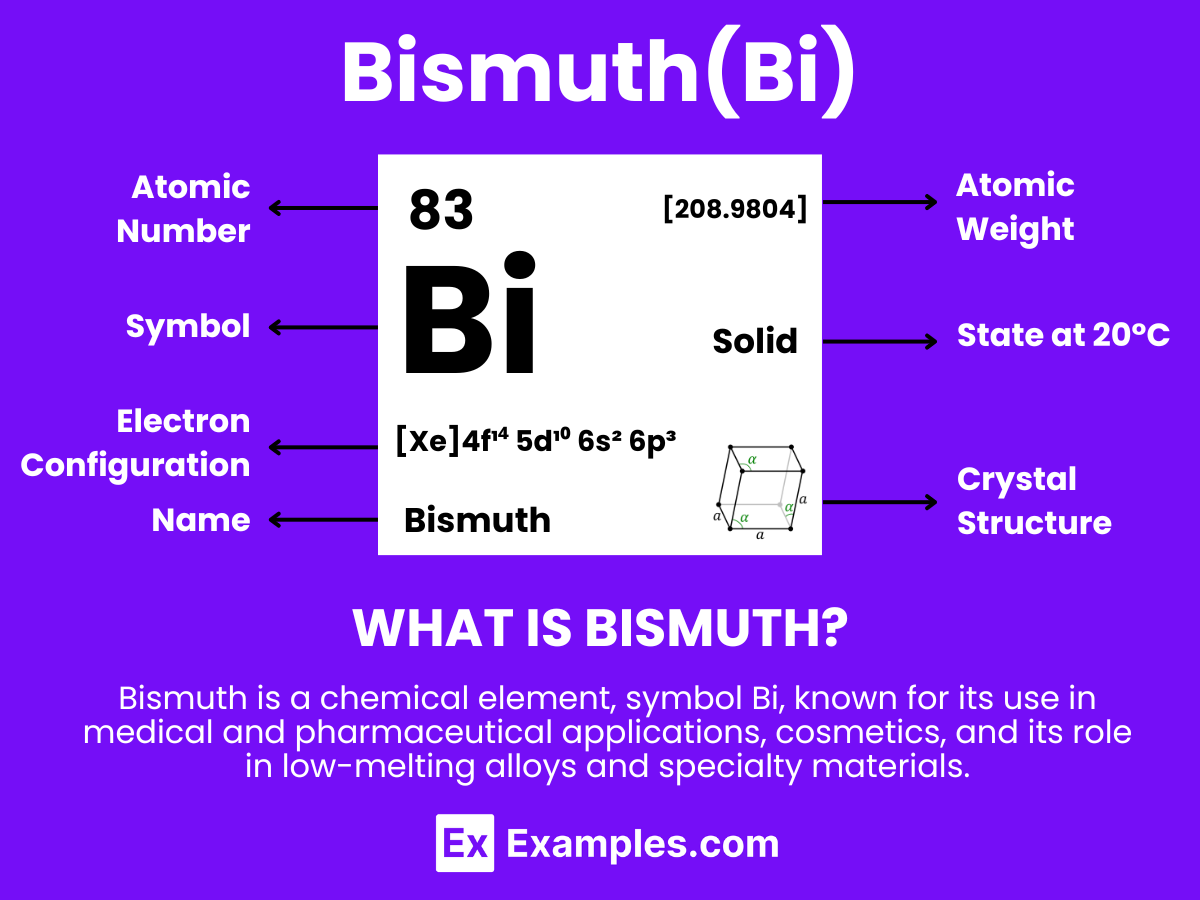
Embark on a journey through the fascinating realm of Bismuth, an element that often seems cloaked in hyperbole due to its colorful appearance and myriad of uses. This all-encompassing guide offers a deep dive into Bismuth, from its definition and types to its practical applications in various industries. With vivid examples, we aim to demystify Bismuth for educators, students, and anyone keen on understanding this versatile element. Unlock the secrets of Bismuth and discover its true potential in our everyday lives.
Bismuth is a chemical element with the symbol Bi and atomic number 83. Known for its rainbow-colored oxide film, bismuth stands out with its distinctive, shiny appearance. It is a brittle metal with a low melting point, making it useful in various applications such as cosmetics, pharmaceuticals, and the manufacturing of low-melting alloys for fire detection and safety devices. Bismuth is non-toxic, which distinguishes it from many other metals, and it is increasingly used as a replacement for lead in various products.
Bismuth, unlike hydrogen, is a non-metallic element known for its unique characteristics, including a stable solid form at room temperature and distinctive properties derived from its status as a post-transition metal.
This structure is based on metallic bonding to a degree, with a significant covalent character due to the sharing of electrons across many bismuth atoms, differing fundamentally from the discrete electron sharing observed in hydrogen’s covalent bonds. Bismuth’s solid form is stable and exhibits low thermal and electrical conductivity compared to true metals, highlighting its unique position among elements with semi-metallic properties.
| Property | Description |
|---|---|
| Appearance | A brittle, silvery metal with a pink tinge. |
| State at Room Temperature | Solid |
| Melting Point | 271.4°C (520.5°F) |
| Boiling Point | 1564°C (2847°F) |
| Density | 9.78 g/cm³ at 20°C |
| Electrical Conductivity | Poor conductor of electricity. |
Bismuth exhibits several important chemical properties:
| Property | Value |
|---|---|
| Melting Point | 271.3°C (520.3°F) |
| Boiling Point | 1564°C (2847°F) |
| Heat of Fusion | 10.9 kJ/mol |
| Heat of Vaporization | 179 kJ/mol |
| Specific Heat Capacity | 25.52 J/(mol·K) |
| Thermal Conductivity | 7.97 W/(m·K) |
| Property | Value |
|---|---|
| Density at 20°C | 9.78 g/cm³ |
| Atomic Mass | 208.98040 u |
| Crystal Structure | Rhombohedral |
| Mohs Hardness | 2.25 |
| Young’s Modulus | 32 GPa |
| Poisson’s Ratio | 0.33 |
| Property | Value |
|---|---|
| Electrical Conductivity | 0.77 × 10^6 S/m |
| Magnetic Susceptibility | -16.6 × 10^-6 cm^3/mol |
| Thermal Conductivity | 7.97 W/(m·K) |
| Property | Value |
|---|---|
| Atomic Number | 83 |
| Isotopes | Bismuth-209 (most stable) |
| Radioactivity | Bismuth-209 is slightly radioactive |
Bismuth, known for its distinctive properties and applications, is typically found and prepared through a distinct set of processes. Here are five key points regarding the preparation process of bismuth:
Bismuth trioxide is one of the most important compounds of bismuth, used in various industrial applications.
Equation: 4Bi + 3O₂ → 2Bi₂O₃
Bismuth dioxide is another oxide of bismuth, demonstrating the metal’s ability to exhibit different oxidation states.
Equation: 4Bi + 4O₂ → 4BiO₂
Bismuth subsalicylate is the active ingredient in many gastrointestinal medications.
Equation: C₇ H₅BiO₄ or [(Bi(C₆H₄OHCO₂)₃]
Bismuth trichloride shows bismuth’s ability to form halides, playing a role in synthetic chemistry.
Equation: 2Bi + 3Cl₂ → 2BiCl₃
Bismuth oxychloride is a compound used in cosmetics, illustrating bismuth’s utility in producing pearlescent pigments.
Equation: BiCl₃ + H₂O → BiOCl + 2HCl
Bismuth hydrate is a hydrated form of bismuth compounds, often encountered in pharmaceutical applications.
Equation: Bi₂O₃ + nH₂O → Bi₂O₃·nH₂O
| Isotope | Half-life | Mode of Decay |
|---|---|---|
| Bismuth-209 | 1.9 × 10^19 years | Alpha decay to Thallium-205 |
| Bismuth-210 | 5.012 days | Beta decay to Polonium-210 |
| Bismuth-211 | 2.14 minutes | Alpha decay to Thallium-207 |
Bismuth’s unique properties, including its low toxicity, high density, and low melting point, make it valuable in a variety of applications:
Bismuth, known for its distinctive rainbow appearance due to surface oxidation, plays a crucial role in a wide range of industrial, scientific, and medical applications. Its non-toxicity and unique physical properties make it particularly valuable in the pharmaceutical industry, low-melting alloys, and cosmetic formulations, illustrating its versatility and importance in modern technology and health care.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical symbol for Bismuth?
Bi
Bs
Bm
Bt
Bismuth is classified as which type of element?
Alkali metal
Transition metal
Post-transition metal
Metalloid
What is the common oxidation state of Bismuth in its compounds?
+1
+2
+3
+4
Which property is unique to Bismuth among the heavy metals?
High density
Low toxicity
High melting point
Radioactivity
Bismuth is used in which type of medical treatment?
Chemotherapy
Antacids
Antibiotics
Vaccines
What is the melting point of Bismuth?
156°C
271°C
321°C
480°C
Which of the following is a notable physical property of Bismuth?
Magnetic
Diamagnetic
Paramagnetic
Ferromagnetic
Which alloy is commonly made using Bismuth?
Brass
Bronze
Pewter
Wood's metal
Bismuth is primarily obtained from which mineral?
Bauxite
Galena
Hematite
Bismuthinite
Which property of Bismuth makes it useful in fire detection systems?
High thermal conductivity
Low melting point
High electrical conductivity
High reflectivity
Before you leave, take our quick quiz to enhance your learning!

