What is brass primarily composed of?
Copper and zinc
Copper and tin
Copper and nickel
Copper and aluminum

Brass is a metal alloy made of copper and zinc. The amounts of these two metals can be adjusted to create different kinds of brass with various properties. It’s known for its shiny gold-like appearance and is used in many things around us. From musical instruments like trumpets and saxophones to door handles, decorations, and even some coins, brass is everywhere. It’s popular because it’s easy to work with, durable, and resists corrosion well, making it ideal for a wide range of uses.
Brass is an alloy, which means it is made by combining two or more metals to create a new material with unique properties. The primary metals in brass are copper and zinc, and its formula varies based on the proportion of these metals. Generally, there isn’t a fixed chemical formula for brass like there is for pure substances because its composition can change. However, a common type of brass might be represented by the formula CuZn, indicating that it primarily consists of copper (Cu) and zinc (Zn).
The structure of brass depends on the ratio of copper to zinc; more zinc makes the brass harder and stronger, while more copper gives it a softer, more malleable quality. This mix allows manufacturers to tailor the brass for specific uses, from flexible wires to sturdy door handles. At the microscopic level, brass’s structure can show a mixture of both copper and zinc crystals, giving it its distinctive properties like corrosion resistance, electrical conductivity, and a shiny, gold-like appearance.
Brass is prepared by melting copper and zinc together in a process known as alloying. The typical method involves heating copper to its melting point in a furnace, then adding zinc to the molten copper. The proportion of copper to zinc can vary, but a common mixture is about 70% copper and 30% zinc for what’s often called ‘cartridge brass’. As the metals melt, they combine to form brass. The chemical reaction can be simplified and represented by the equation:
This is a straightforward representation since the actual process doesn’t involve a simple chemical reaction but rather the physical mixing and fusion of two metal atoms at high temperatures. Once the brass is formed, it is cooled and solidified into the desired shape for use in various applications, from musical instruments to plumbing fixtures.
| Property | Description |
|---|---|
| Color | Brass has a bright, gold-like appearance, which can vary from reddish to yellow depending on the copper to zinc ratio. |
| Hardness | Generally harder than copper due to the presence of zinc, but the exact hardness can vary based on the specific type of brass. |
| Malleability | Can be easily shaped and formed, especially when heated, making it ideal for crafting intricate designs. |
| Conductivity | Good electrical and thermal conductivity, though not as high as pure copper, making it useful for certain electrical and heat-related applications. |
| Corrosion Resistance | Resists corrosion from water and many chemicals, making it suitable for marine and outdoor uses. |
| Melting Point | Brass has a lower melting point than copper, typically around 900°C to 940°C (1652°F to 1724°F), facilitating easier casting and molding. |
| Density | Slightly less dense than copper, with an average density of about 8.4 to 8.73 grams per cubic centimeter. |
One of the key chemical properties of brass is its resistance to corrosion, making it ideal for use in marine environments and for plumbing fixtures. Brass forms a protective layer of zinc carbonate (ZnCO3) when exposed to carbon dioxide (CO2) and moisture, which helps protect the underlying metal from further corrosion.
Brass can tarnish when exposed to air containing sulfur compounds, leading to a darker surface appearance. This is due to the formation of copper sulfide (Cu2S) and zinc sulfide (ZnS) on the surface.
Brass exhibits antimicrobial properties, especially against bacteria and viruses. The copper content in brass can disrupt microbial cell membranes and inactivate the microorganisms, making brass surfaces self-sanitizing over time. This property is particularly valued in healthcare settings.
Brass can be weakened by ammonia (NH3) and ammonium compounds, causing a phenomenon known as “season cracking.” This is due to the formation of complex ions with zinc, leading to stress corrosion cracking in the presence of tensile stress
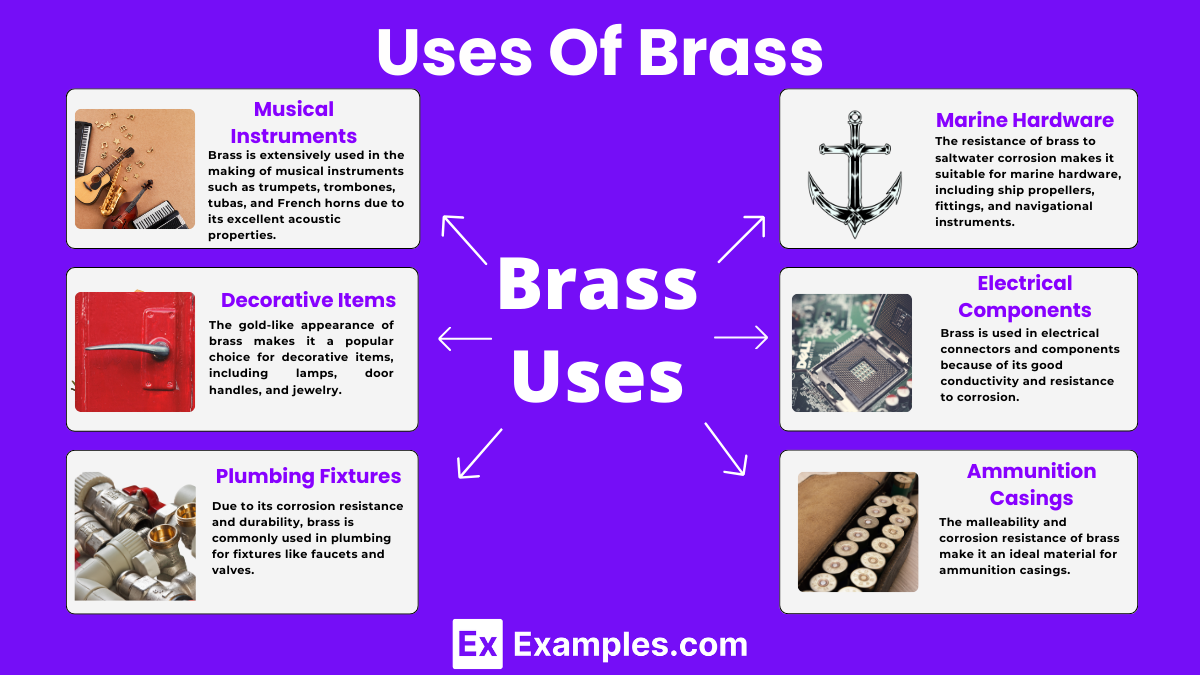
Brass is extensively used in the making of musical instruments such as trumpets, trombones, tubas, and French horns due to its excellent acoustic properties. The material helps in producing rich, vibrant sounds that are characteristic of brass instruments.
The gold-like appearance of brass makes it a popular choice for decorative items, including lamps, door handles, and jewelry. Its ability to resist corrosion and tarnish adds to the aesthetic appeal and longevity of these items.
Due to its corrosion resistance and durability, brass is commonly used in plumbing for fixtures like faucets and valves. It ensures a longer lifespan and maintains water quality by inhibiting microbial growth.
The resistance of brass to saltwater corrosion makes it suitable for marine hardware, including ship propellers, fittings, and navigational instruments. This ensures the safety and durability of marine equipment.
Brass is used in electrical connectors and components because of its good conductivity and resistance to corrosion. These properties ensure reliable and efficient electrical connections in various applications.
The malleability and corrosion resistance of brass make it an ideal material for ammunition casings. It can withstand the expansion and contraction during firing, ensuring safety and reliability.
The antimicrobial properties of brass are utilized in healthcare and public settings to reduce the transmission of infections. Door handles, push plates, and fixtures made of brass help in minimizing microbial contamination.
| Property | Brass | Bronze |
|---|---|---|
| Main Components | Primarily copper and zinc. | Primarily copper and tin, often with other elements like aluminum, manganese, nickel, or zinc added. |
| Color | Ranges from bright yellow to reddish-yellow, depending on the zinc content. | Typically a dull gold to reddish-brown, varying with the specific alloy composition. |
| Hardness | Generally softer than bronze, making it easier to machine and work with. | Harder than brass, offering greater resistance to wear and tear. |
| Conductivity | Good electrical conductivity due to high copper content, but generally less than that of pure copper. | Lower electrical conductivity compared to brass, due to the addition of tin and other elements. |
| Corrosion Resistance | Good resistance to corrosion, particularly from water. Forms a protective patina that can protect against further corrosion. | Excellent corrosion resistance, especially in marine environments, due to the formation of a protective surface layer. |
| Applications | Widely used in musical instruments, decorative items, plumbing fittings, and electrical components. | Commonly used in marine applications, statues, bearings, and gears due to its strength and resistance to sea water. |
| Cost | Typically less expensive than bronze, due to the lower cost of zinc compared to tin. | Generally more expensive than brass, because tin is more costly than zinc. |
Brass’s durability makes it ideal for long-lasting applications, reducing the need for replacements.
Its resistance to corrosion, especially from water, ensures longevity and reliability in various environments.
Brass’s bright, gold-like appearance enhances the aesthetic value of decorative items and architectural features.
Natural antimicrobial properties make brass a hygienic choice, limiting the spread of bacteria and viruses.
Brass offers efficient thermal and electrical conductivity, suitable for heating systems and electrical components.
Its malleability allows for easy shaping and molding, facilitating intricate designs and manufacturing processes.
100% recyclability makes brass an environmentally friendly material, supporting sustainable practices.
In slang, “brass” often refers to brass instruments in a band or, metaphorically, to audacity or boldness.
Brass is not just copper; it’s an alloy combining copper and zinc, which gives it distinct properties.
Copper and zinc create brass, not gold. No combination of metals can chemically produce gold.
Brass can turn green due to corrosion, forming a patina when exposed to moisture over time
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is brass primarily composed of?
Copper and zinc
Copper and tin
Copper and nickel
Copper and aluminum
Which property of brass makes it suitable for use in musical instruments?
High electrical conductivity
Malleability and acoustic properties
High melting point
Resistance to corrosion
Which of the following is a common use of brass in household items?
Electrical wiring
Plumbing fixtures
Food containers
Roofing materials
What is the primary advantage of using brass in marine applications?
High strength
Corrosion resistance
Low cost
High melting point
What color is brass typically?
Silver
Gray
Yellow-gold
White
How does the zinc content in brass affect its properties?
Increases its density
Decreases its melting point
Enhances its corrosion resistance
Makes it brittle
Which process is commonly used to shape brass into desired forms?
Casting
Electroplating
Sintering
Distillation
What is the melting point range of brass?
800-900°C
900-1000°C
1000-1100°C
1100-1200°C
Which alloying element in brass improves its machinability?
Tin
Lead
Nickel
Aluminum
What is the term for the process of forming brass sheets into thin, flat pieces?
Rolling
Forging
Extruding
Annealing
Before you leave, take our quick quiz to enhance your learning!

