What is the atomic number of Cerium?
56
57
58
59
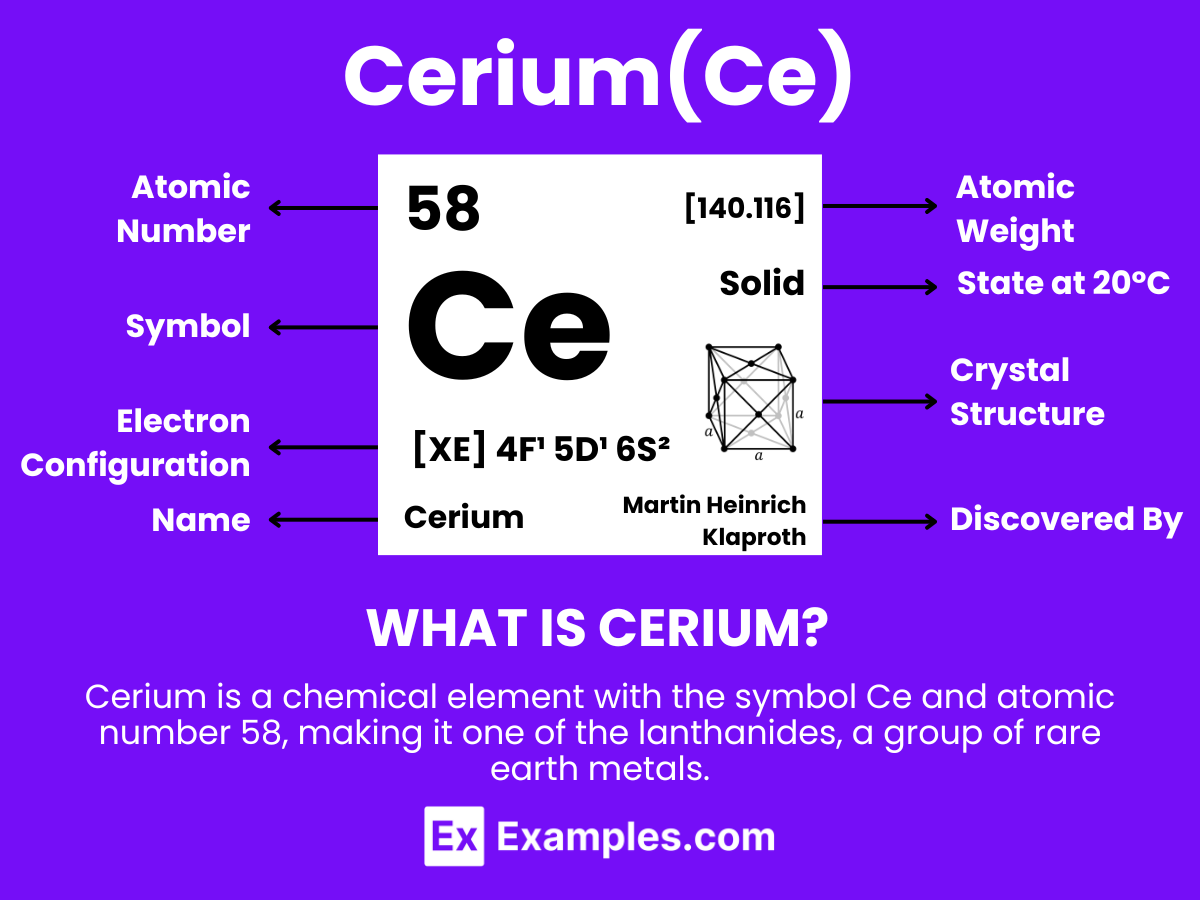
Dive into the comprehensive guide on Cerium, a cornerstone of the lanthanide series with far-reaching applications and fascinating chemistry. This guide unveils the essence of Cerium, from its basic definition to its multifaceted roles in enhancing modern technologies and its intriguing compounds. With practical examples, you’ll explore how Cerium contributes to environmental sustainability, medical advancements, and the technology sector, showcasing its indispensable presence in our daily lives and the future of innovation.
Cerium is a chemical element with the symbol Ce and atomic number 58, making it one of the lanthanides, a group of rare earth metals. It is a soft, silvery, ductile metal that tarnishes when exposed to air and is both malleable and relatively hard. Cerium has the distinction of being the most abundant of the rare earth elements, found in a variety of minerals including cerite, monazite, bastnäsite, and others.Cerium has notable chemical properties; it is especially reactive, easily oxidizing in the air and reacting with water. It exists in two oxidation states: +3 and +4, with the +4 state being more stable in oxygenated environments. This dual oxidative capacity enables cerium to act as a strong oxidizing agent in the +4 state, while in the +3 state, it behaves more like a typical rare earth metal.
Cerium (Ce) is an intriguing element in the lanthanide series of the periodic table, showcasing a unique atomic structure that underpins its diverse chemical behavior and applications. Here are the key aspects of Cerium’s atomic structure:
| Property | Value |
|---|---|
| Appearance | Silvery-white, malleable, ductile metal |
| Atomic Mass | 140.116 u |
| Density | 6.77 g/cm³ at 20 °C |
| Melting Point | 798 °C |
| Boiling Point | 3360 °C |
| Atomic Number | 58 |
| Atomic Radius | 185 pm |
| State at 20 °C | Solid |
| Crystal Structure | Face-centered cubic (fcc) |
| Electrical Resistivity | 828 nΩ·m at 20 °C |
| Thermal Conductivity | 11.3 W/(m·K) at 25 °C |
| Thermal Expansion | 6.3 µm/(m·K) at 25 °C |
Cerium, a rare earth metal, exhibits fascinating chemical properties due to its position in the lanthanide series. Its most common oxidation states are +3 (Ce³⁺) and +4 (Ce⁴⁺), with the +4 state being more stable in aqueous solutions. Cerium’s chemistry is characterized by its ability to readily switch between these oxidation states, which is utilized in various chemical reactions and applications.
Oxidation and Reduction:
Cerium can easily oxidize from the +3 to the +4 oxidation state, especially in aqueous solutions. This property makes it a strong oxidizing agent.
Ce³⁺ (aq) → Ce⁴⁺⁻
Conversely, Ce^4+ can act as an oxidizer, getting reduced to Ce^3+ in redox reactions.
Ce⁴⁺⁻ → Ce³+(aq)
Reaction with Water:
Cerium reacts with water, forming cerium(III) hydroxide and releasing hydrogen gas, especially when finely divided.
2Ce + 6H2O → 2Ce(OH)₃ + 3H2↑
Reaction with Acids:
Cerium metal dissolves in dilute acids, forming Ce³⁺ solutions.
Ce + 2HCl → CeCl₂ + H2↑
In more oxidizing acids like nitric acid, cerium can form Ce⁴⁺.
2Ce + 6HNO₃ → 2Ce(NO₃)₄ + 3H2O + 3NO2↑
Reaction with Oxygen:
At room temperature, cerium slowly tarnishes, forming a thin layer of cerium oxide (CeO₂), which prevents further oxidation.
4Ce + O2 → 2Ce2O₃
Upon heating, cerium burns vigorously to form CeO₂.
2Ce + O2 → 2CeO₂
Formation of Cerium Compounds:
Cerium forms a variety of compounds, both in +3 and +4 oxidation states, including oxides (Ce₂O₃ and CeO₂), sulfides (Ce₂S₃), and halides (CeF₃, CeCl₃, CeBr₃, CeI₃, etc.).
CeO₂, in particular, is widely used as a catalyst in automotive exhaust systems to reduce emissions and in chemical synthesis.
| Property | Value |
|---|---|
| Standard Atomic Weight | 140.116 u |
| Enthalpy of Fusion | 5.46 kJ/mol (at melting point) |
| Enthalpy of Vaporization | 398 kJ/mol (at boiling point) |
| Enthalpy of Atomization | 418 kJ/mol (at 25 °C) |
| Specific Heat Capacity | 26.94 J/(mol·K) (at 25 °C) |
| Thermal Conductivity | 11.3 W/(m·K) (at 25 °C) |
| Thermal Expansion | 6.3 µm/(m·K) (at 25 °C) |
| Property | Value |
|---|---|
| Young’s Modulus | 33.6 GPa |
| Shear Modulus | 13.5 GPa |
| Bulk Modulus | 21.5 GPa |
| Poisson’s Ratio | 0.24 |
| Mohs Hardness | 2.5 |
| Vickers Hardness | 270 MPa |
| Brinell Hardness | 412 MPa |
| Density | 6.77 g/cm³ (at 20 °C) |
| Property | Value |
|---|---|
| Electrical Resistivity | 828 nΩ·m (at 20 °C) |
| Magnetic Ordering | Paramagnetic (above 12 K) |
| Magnetic Susceptibility | +2730.0·10⁻⁶ cm³/mol (at 298 K) |
| Electrical Conductivity | About 1.25·10⁶ S/m |
| Property | Value |
|---|---|
| Natural Isotopes | ¹³⁶Ce, ¹³⁸Ce, ¹⁴⁰Ce, ¹⁴²Ce |
| Most Stable Isotopes | ¹⁴⁰Ce (stable), ¹⁴²Ce (stable), ¹³⁸Ce (stable), ¹³⁶Ce (stable) |
| Isotopic Abundance | ¹⁴⁰Ce (88.48%), ¹⁴²Ce (11.08%), ¹³⁸Ce (0.25%), ¹³⁶Ce (0.19%) |
| Neutron Cross Section | ¹⁴⁰Ce: 0.57 barns (thermal neutrons) |
| Neutron Mass Absorption | 0.00013 |
| Atomic Number | 58 |
| Half-life of Most Unstable Isotope | ¹⁵²Ce (half-life: ~8.4 years) |
Cerium is predominantly obtained from cerium-rich minerals like monazite and bastnasite, which contain a mixture of rare earth elements. The extraction and preparation process involves several steps:
1.Cerium(III) Oxide (Ce₂O₃):
2.Cerium(IV) Oxide (CeO₂):
3.Cerium(III) Chloride (CeCl₃):
4.Cerium(IV) Sulfate (Ce(SO₄)₂):
5.Cerium(III) Nitrate (Ce(NO₃)₃):
6.Cerium(IV) Bromide (CeBr₄CeBr₄):
| Isotope | Natural Abundance (%) | Half-life | Decay Mode |
|---|---|---|---|
| ¹³⁶Ce | 0.185 | Stable | – |
| ¹³⁸Ce | 0.251 | Stable | – |
| ¹⁴⁰Ce | 88.450 | Stable | – |
| ¹⁴²Ce | 11.114 | Stable | – |
| ¹⁴⁶Ce | – | 284.893 days | Beta decay to ¹⁴⁴Pr |
Cerium, a versatile and abundant rare earth element, has a wide range of applications across various industries due to its unique physical and chemical properties. Some of the primary uses of cerium include:
The production of cerium involves several key processes, beginning with the extraction from rare earth mineral ores, such as monazite and bastnasite, which contain a mix of cerium and other lanthanides. The basic steps in cerium production include:
Cerium and its compounds have a wide range of applications, reflecting the metal’s versatile properties:
Cerium, a versatile and abundant element in the Earth’s crust, plays a crucial role in modern technology and industry. Its unique physical, chemical, and nuclear properties enable its use in catalysis, glass polishing, and as an alloying agent, among others. Understanding cerium’s isotopes, including both stable and radioactive varieties, provides insight into its applications and environmental impact.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Cerium?
56
57
58
59
What is the chemical symbol for Cerium?
Ce
Cr
Cs
Cm
Cerium belongs to which group of elements?
Transition metals
Alkali metals
Lanthanides
Actinides
What is the most common oxidation state of Cerium in its compounds?
+1
+2
+3
+4
Which mineral is the primary source of Cerium?
Bauxite
Galena
Hematite
Monazite
Which of the following is a common use of Cerium oxide?
Fertilizers
Water purification
Abrasives in polishing
Building materials
Cerium is used in which component of automotive catalytic converters?
Catalyst support
Exhaust pipes
Muffler
Fuel tank
What is the melting point of Cerium?
798°C
795°C
840°C
650°C
Cerium is used in flints for which purpose?
Enhancing durability
Increasing flexibility
Preventing rust
Producing sparks
Which of the following is NOT a property of Cerium?
Soft and malleable
Silvery-white
Highly reactive
Brittle
Before you leave, take our quick quiz to enhance your learning!

