What is the atomic number of Cobalt?
26
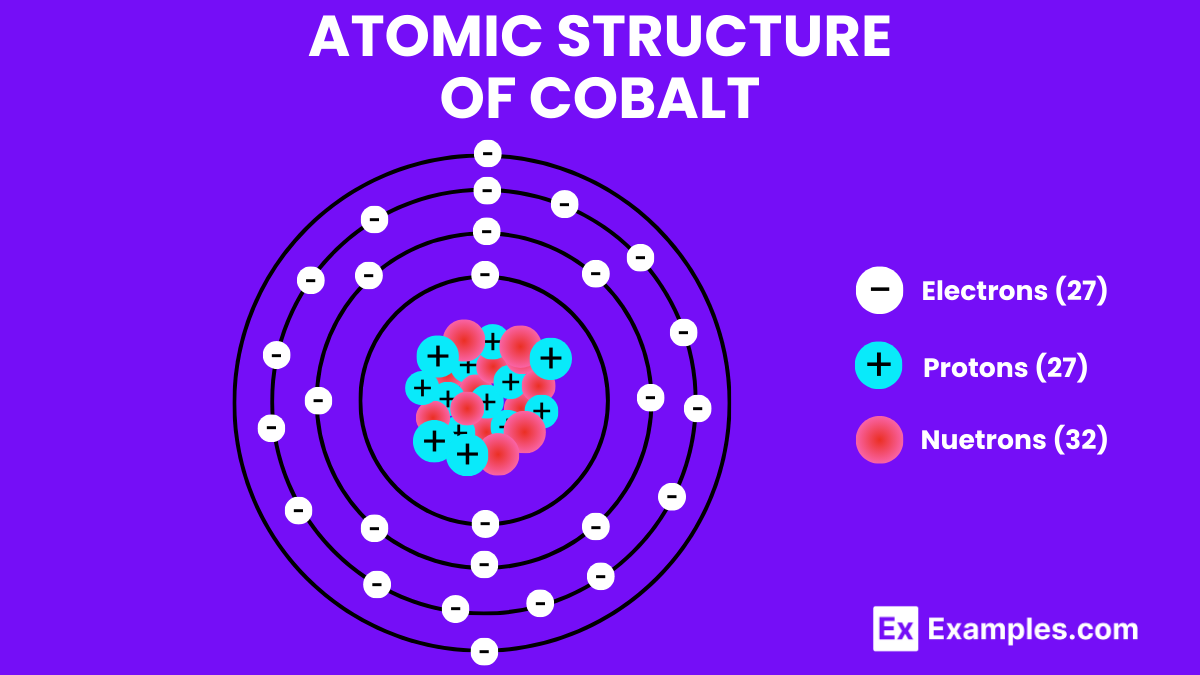
27
28
29
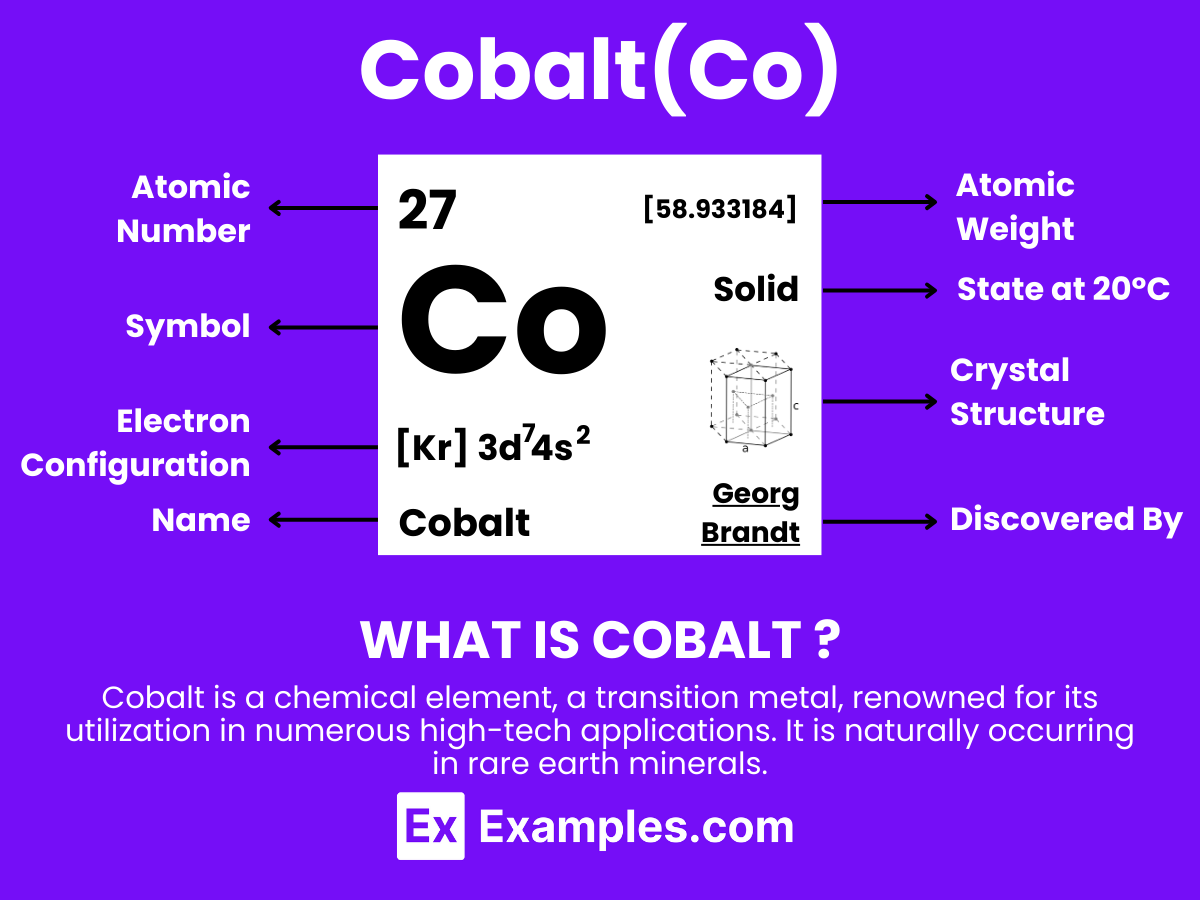
Cobalt, a transition metal nestled in the heart of the periodic table, is pivotal to modern technology and green energy solutions. This guide uncovers the multifaceted roles cobalt plays, from its use in rechargeable batteries to its significance in alloy production. With detailed examples, we’ll explore how cobalt’s unique properties fuel advancements in electronics, healthcare, and sustainable energy. Dive into the vibrant world of cobalt and discover its critical contributions to innovation and environmental sustainability.
| Property | Value |
|---|---|
| Appearance | Lustrous, metallic, silver-gray |
| Atomic Number | 27 |
| Atomic Mass | 58.933195 u |
| Density at 20°C | 8.90 g/cm³ |
| Melting Point | 1495°C (2723°F) |
| Boiling Point | 2927°C (5301°F) |
| State at 20°C | Solid |
| Electrical Conductivity | 17.2 × 10^6 S/m |
| Thermal Conductivity | 100 W/(m·K) |
| Heat of Fusion | 16.06 kJ/mol |
| Heat of Vaporization | 377 kJ/mol |
| Specific Heat Capacity | 24.81 J/(mol·K) |
Cobalt, symbolized as Co, is a transition metal with a series of unique chemical properties, allowing it to participate in a variety of chemical reactions. Its ability to exist in multiple oxidation states, especially +2 and +3, plays a crucial role in its chemical behavior.
Cobalt commonly exhibits two oxidation states in its compounds:
Reactivity with Oxygen: Cobalt reacts with oxygen to form cobalt(II) oxide at standard temperatures, and further heating can lead to the formation of cobalt(III) oxide. 4Co+3O₂→2Co₂O₃
Reactivity with Acids: Cobalt reacts with dilute sulfuric acid or hydrochloric acid to produce cobalt(II) salts and hydrogen gas. 2Co+2HCl→CoCl₂+H₂
Reactivity with Water: Cobalt is relatively stable in water but can oxidize over time, especially in the presence of oxygen, forming a thin layer of cobalt oxide on its surface.
Thermal Stability: Cobalt compounds exhibit high thermal stability, a property that is utilized in high-temperature applications and materials.
Role in Catalysis: Cobalt catalysts are used in several industrial processes, including the Fischer-Tropsch synthesis for converting syngas into liquid hydrocarbons. Cobalt catalysts facilitate the hydrogenation of carbon monoxide to produce various hydrocarbons.
Magnetic Properties: While not a chemical reaction, it’s notable that cobalt compounds, especially those in the +2 oxidation state, exhibit magnetic properties. This characteristic is essential in the development of magnetic materials and devices.
Environmental and Biological Role: Cobalt is a key component of vitamin B12, essential for human health. However, cobalt ions can be toxic in large amounts, indicating the need for careful handling and monitoring of cobalt-containing compounds.
| Property | Value |
|---|---|
| Melting Point | 1495°C (2723°F) |
| Boiling Point | 2927°C (5301°F) |
| Heat of Fusion | 16.06 kJ/mol |
| Heat of Vaporization | 377 kJ/mol |
| Specific Heat Capacity | 24.81 J/(mol·K) |
| Thermal Conductivity | 100 W/(m·K) |
| Thermal Expansion | 13.0 µm/(m·K) (at 25°C) |
| Property | Value |
|---|---|
| Atomic Mass | 58.933195 u |
| Density | 8.90 g/cm³ (at room temperature) |
| Mohs Hardness | 5 |
| Young’s Modulus | 209 GPa |
| Bulk Modulus | 180 GPa |
| Poisson’s Ratio | 0.31 |
| Property | Value |
|---|---|
| Electrical Resistivity | 6.24 µΩ·m (at 20°C) |
| Magnetic Ordering | Ferromagnetic |
| Curie Temperature | 1115°C (2039°F) |
| Magnetic Susceptibility | High |
| Property | Value |
|---|---|
| Isotopes | ^59Co (Stable) |
| Atomic Number | 27 |
| Atomic Weight | 58.933195 |
| Half-life of Most Stable Isotope (^60Co) | 5.2714 years |
| Neutron Cross Section | 37.2 barns (for ^59Co) |
| Neutron Mass Absorption | 0.00245 |
| Isotope | Mass Number | Natural Abundance (%) | Half-Life | Notes |
|---|---|---|---|---|
| Co-59 | 59 | 100 | Stable | Only stable isotope, prevalent in nature |
| Co-60 | 60 | Synthetic | 5.2714 years | Used in radiotherapy and as a radiation source in industry |
| Co-57 | 57 | Synthetic | 271.79 days | Utilized in medical diagnostic tests, especially in nuclear medicine |
Cobalt’s remarkable properties find use in a variety of applications, making it an indispensable element in modern technology and industry.
Our exploration of cobalt reveals its indispensable role in modern technology and sustainable solutions. The detailed table illustrates cobalt’s unique physical and chemical properties, underscoring its significance in batteries, alloys, and catalysts. Understanding cobalt’s attributes and its meticulous preparation processes highlights the metal’s pivotal contributions to advancing green energy and high-performance materials, marking it as a cornerstone of innovation.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Cobalt?
26
27
28
29
What is the chemical symbol for Cobalt?
Co
Cu
Cd
Cr
Cobalt is primarily obtained from which type of ore?
Hematite
Bauxite
Pentlandite
Cobaltite
What is a common oxidation state of Cobalt in its compounds?
+1
+2
+3
+4
Which of the following is a primary use of Cobalt in industry?
Fertilizers
Batteries
Plastics
Textiles
Which alloy contains Cobalt and is known for its high strength and temperature resistance?
Stainless steel
Bronze
Stellite
Brass
What color does Cobalt typically impart to glass and ceramics?
Red
Green
Blue
Yellow
Which isotope of Cobalt is commonly used in radiotherapy for cancer treatment?
Co-58
Co-59
Co-60
Co-61
Cobalt is a part of which block in the periodic table?
s-block
p-block
d-block
f-block
What is the density of Cobalt?
7.0 g/cm³
8.9 g/cm³
10.5 g/cm³
12.3 g/cm³
Before you leave, take our quick quiz to enhance your learning!

