What is the atomic number of copernicium?
110
112
114
116
Dive into the world of superheavy elements with our complete guide to Copernicium, a fascinating member of the periodic table’s elite. This detailed exploration offers insights into Copernicium’s discovery, properties, and potential applications, showcasing its unique position among the giants of modern chemistry. Through engaging examples and expert analysis, we unveil the mysteries surrounding this elusive element, enriching your understanding of the ever-expanding periodic table. Join us on a journey into the atomic depths, where Copernicium lies in wait, ready to reveal its secrets.
What is Copernicium ?
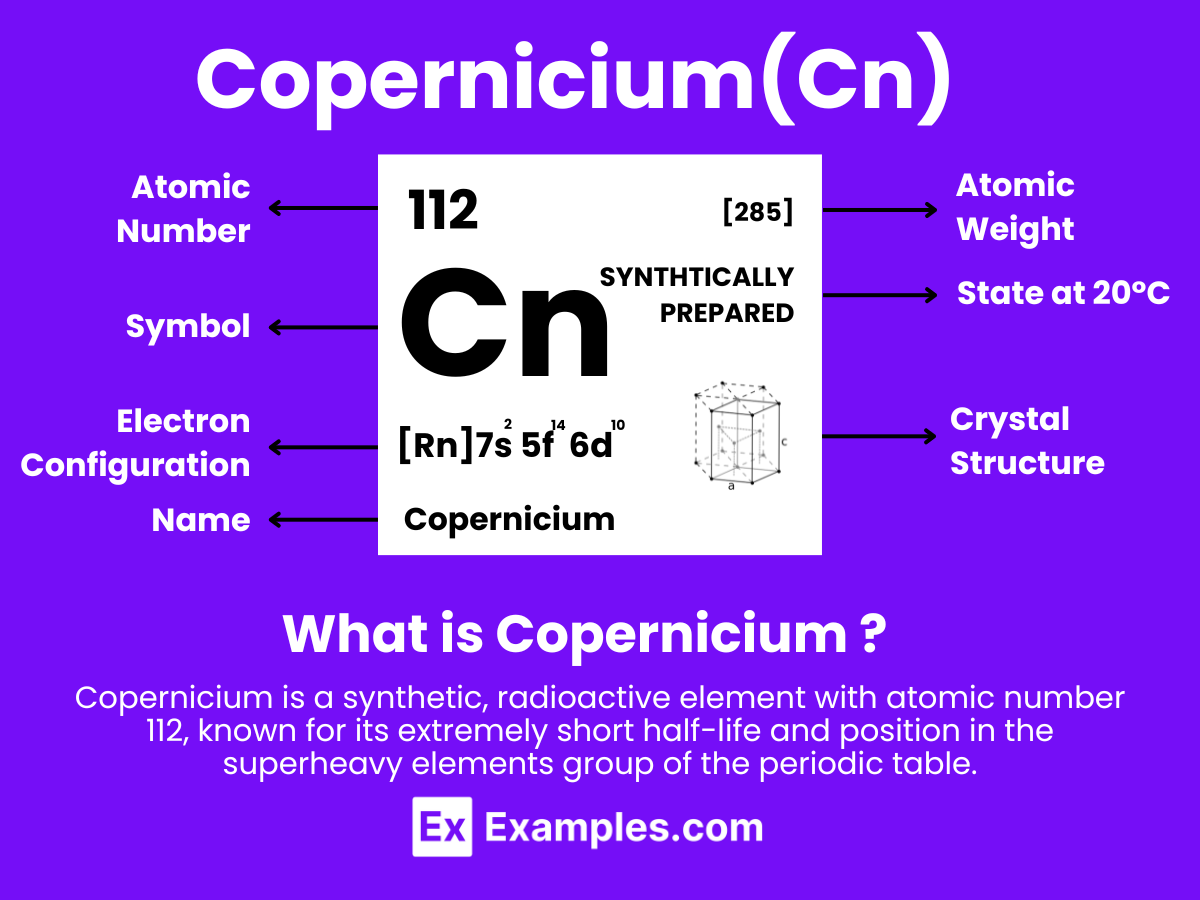
Copernicium is a synthetic chemical element with the symbol Cn and atomic number 112. It belongs to group 12 of the periodic table and is a transactinide element. Copernicium was first created in 1996 by the GSI Helmholtz Centre for Heavy Ion Research in Darmstadt, Germany. The element is named after the astronomer Nicolaus Copernicus, in recognition of his contribution to the understanding of our solar system’s heliocentric model.
Copernicium is a highly radioactive metal that has only been produced in minute amounts and has no stable isotopes. The most stable known isotope, copernicium-285, has a half-life of approximately 29 seconds, although there are claims of isotopes with longer half-lives. This short existence makes it challenging to study, and as such, much of what is known about copernicium comes from theoretical calculations rather than direct experimental data.
Formula: Cn
Composition: Consists of a single copernicium atom.
Bond Type: In its elemental form, copernicium does not have bonds as it is a pure element. However, copernicium can form covalent or ionic bonds when reacting with other elements.
Molecular Structure: As a pure element, copernicium does not form a molecular structure in the same sense as compounds. Theoretical predictions suggest it might display a metallic state with an unknown crystalline structure due to its position in the periodic table.
Electron Sharing: In compounds, copernicium is expected to share electrons covalently or transfer electrons ionically, depending on the nature of the other element(s) it is bonding with, although specific compound examples are largely theoretical due to its short half-life.
Significance: Copernicium is notable for being a superheavy element with a very short half-life, limiting its practical applications but making it of great interest in nuclear physics and the study of the periodic table’s limits.
Role in Chemistry: Copernicium’s role in chemistry is primarily theoretical and research-oriented, given its synthetic nature and extremely limited availability. It is used to explore the chemical and physical properties of superheavy elements, contributing to our understanding of the periodic table’s heaviest members.
Copernicium, unlike hydrogen, is a metal with theoretical characteristics that suggest it would exhibit highly unusual properties, including potential volatility and a possibly short existence in solid or liquid form due to its radioactive nature. Copernicium’s behavior at the atomic and molecular levels is vastly different from that of hydrogen, owing to its position in the periodic table as a superheavy element and its predicted metallic nature.
Atomic Level: Each copernicium atom (Cn) contains 112 protons in its nucleus and is expected to have 112 electrons orbiting around it. The electron configuration of copernicium is predicted to be [Rn] 5f¹⁴ 6d¹⁰ 7s², indicating it has two electrons in its outermost shell, suggesting a stability in its +2 oxidation state similar to the lighter group 12 elements.
Molecular Formation: In its metallic form, copernicium would not form molecules in the same manner as H₂. Instead, theoretical predictions suggest that copernicium atoms might be arranged in an unknown crystalline lattice structure if it were possible to observe it in solid form. This structure would involve a metallic bonding scenario where electrons are shared among many copernicium atoms, distinct from the covalent bonding seen in hydrogen molecules. Given its highly radioactive nature and very short half-life, any solid or liquid form of copernicium would be transient and difficult to study directly.
Copernicium’s bonds within any hypothetical lattice are speculative, with predictions suggesting a complex interaction influenced by relativistic effects due to the high atomic number. Unlike hydrogen, which is a gas at room temperature, copernicium’s state under normal conditions is largely theoretical due to its extreme radioactivity and short half-life, making practical observations of its physical state challenging. It is expected that copernicium, if it could be observed in significant quantities, would have no stable or naturally occurring form at room temperature and would likely decay too quickly to measure physical properties such as melting or boiling points accurately.
| Property | Description |
|---|---|
| Atomic Number | 112 |
| Phase at Room Temperature | Expected to be a gas or a volatile liquid, drawing parallels with mercury. |
| Density | Theoretically very dense if it could be observed as a bulk material. |
| Melting Point | Predicted to have a relatively low melting point for metals, possibly making it a liquid at room temperature. |
| Boiling Point | The boiling point is theorized to be extremely high, suggesting strong atomic bonds. |
| Electron Configuration | Predicted to be [Rn] 5f¹⁴ 6d¹⁰ 7s², hinting at unique physical and possibly chemical characteristics. |
The chemical properties of Copernicium (Cn) are largely theoretical due to its short half-life and the difficulty in producing sufficient quantities for experimentation. However, based on its position in the periodic table and predictions from relativistic quantum chemistry, several chemical properties can be inferred:
| Property | Value (Predicted) |
|---|---|
| Atomic Number | 112 |
| Atomic Mass | [285] u |
| Phase at Room Temperature | Expected to be a gas or possibly a volatile liquid |
| Melting Point | Unknown, but speculated to be low for a metal |
| Boiling Point | Predicted to be around 357 K (84 °C; 183 °F) |
| Density | Unknown; estimated to be less than that of mercury |
| Heat of Vaporization | High, indicative of strong metallic bonding |
| Thermal Conductivity | Expected to be low, consistent with other heavy metals |
| Property | Value (Predicted) |
|---|---|
| Atomic Number | 112 |
| Atomic Mass | [285] u |
| State at Room Temperature | Expected to be a gas or volatile liquid |
| Density | Predicted to be low compared to other heavy metals |
| Color | Not observed; speculated to have a metallic appearance |
| Hardness | Not measurable, but theorized to be relatively soft |
| Property | Value (Predicted) |
|---|---|
| Electrical Conductivity | Presumed to be poor due to its gaseous or liquid state |
| Magnetic Susceptibility | Expected to be diamagnetic, like mercury |
| Reflectivity | Theoretical, assumed to be high if it were solid |
| Ionization Energy | High, typical for heavy, p-block elements |
| Property | Value (Predicted/Measured) |
|---|---|
| Half-life of Most Stable Isotope (Cn-285) | Approximately 29 seconds |
| Decay Modes | Alpha decay, leading to lighter elements |
| Isotopes | Known isotopes range from Cn-277 to Cn-285 |
| Neutron to Proton Ratio | High, necessary for stability in superheavy elements |
| Production Method | Cold fusion reactions, typically involving lead targets |
The preparation of Copernicium (Cn) involves highly specialized nuclear reactions, as it is a synthetic element that does not occur naturally. Copernicium is produced in particle accelerators through the collision of lighter atomic nuclei. Here’s an overview of the steps involved in the preparation of Copernicium:
1. Selection of Target and Projectile:
2. Nuclear Reaction:
3. Detection and Isolation:
4. Purification:
| Isotope | Half-life | Decay Modes | Discovery Year | Notes |
|---|---|---|---|---|
| Cn-277 | 0.69 ms | Alpha decay | 1996 | First observed isotope, very short half-life. |
| Cn-281 | 0.1 s | Alpha decay, possibly spontaneous fission | 1999 | Provides insights into the stability of heavier isotopes. |
| Cn-282 | 0.8 ms | Alpha decay | 2002 | Highlighted the island of stability concept. |
| Cn-283 | 4 s | Alpha decay | 2002 | Among the longer-lived isotopes, suggesting increased stability. |
| Cn-284 | 97 ms | Alpha decay | 2002 | Demonstrates the predicted increased stability near the “island of stability”. |
| Cn-285 | 29 s | Alpha decay | 2010 | One of the longest-lived isotopes, significant for theoretical models. |
| Cn-286 | 8.45 s | Alpha decay, possibly electron capture | 2010 | Supports theories on nuclear structure and stability at high atomic numbers. |
Copernicium (Cn) is a synthetic element that does not occur naturally. It is produced in particle accelerators through the fusion of smaller atomic nuclei. The production process involves highly sophisticated equipment and precise conditions to facilitate the necessary nuclear reactions. Here are the primary methods used to produce copernicium:
As of the latest research, copernicium has no practical applications due to its extreme rarity, short half-life, and the complexity involved in its production. Its applications are confined to scientific research, primarily in the fields of nuclear physics and chemistry. The study of copernicium helps scientists:
he exploration of copernicium, a synthetic element with ephemeral existence, underscores the relentless human pursuit of knowledge at the atomic frontier. Through advanced production techniques and speculative applications, copernicium serves as a beacon in nuclear physics and chemistry, illuminating the path toward understanding superheavy elements and the outer limits of the periodic table.
Text prompt
Add Tone
Isotopes of Copernicium
Uses of Copernicium
What is the atomic number of copernicium?
110
112
114
116
Copernicium is classified as which type of element?
Alkali metal
Noble gas
Transition metal
Actinide
What is the symbol for copernicium on the periodic table?
Co
Cp
Cn
Ce
Copernicium was first synthesized in which year?
1996
1998
2000
2002
What is the most stable isotope of copernicium?
Cn-277
Cn-283
Cn-285
Cn-289
Copernicium is named after which famous scientist?
Galileo Galilei
Isaac Newton
Nicolaus Copernicus
Albert Einstein
In which group of the periodic table is copernicium found?
Group 12
Group 13
Group 14
Group 15
What type of element is copernicium?
Metal
Metalloid
Non-metal
Noble gas
Copernicium is expected to have similar chemical properties to which element?
Iron
Gold
Mercury
Lead
What is the predicted density of copernicium?
10 g/cm³
14 g/cm³
23 g/cm³
29 g/cm³
Before you leave, take our quick quiz to enhance your learning!

