What is the atomic number of dubnium?
104
105
106
107
Dive into the world of Dubnium, a lesser-known marvel of the periodic table, through our detailed guide. Dubnium, with its unique properties and synthetic origins, stands as a testament to human ingenuity in the field of chemistry. This guide unfolds the definition, uses, and intriguing aspects of Dubnium, offering insightful examples to illuminate its role in scientific advancements. Join us on a journey to understand Dubnium’s place in modern science, exploring its characteristics, applications, and the compounds it forms.
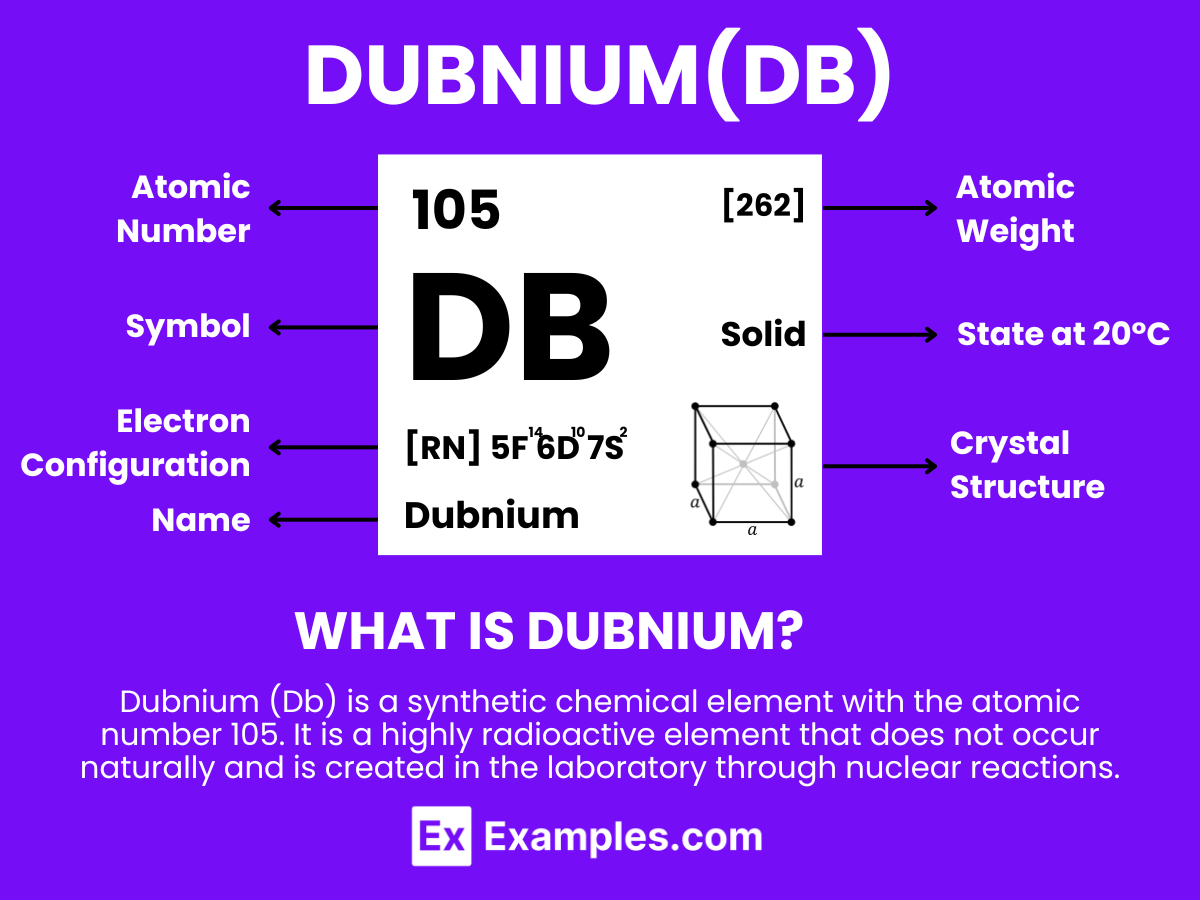
Dubnium (Db) is a synthetic chemical element with the atomic number 105. It is a highly radioactive element that does not occur naturally and is created in the laboratory through nuclear reactions. Named after the Russian town of Dubna, where it was first discovered, dubnium is part of the group of elements known as the transactinides. Because of its instability and short half-life, detailed studies on dubnium are limited, and it has no known practical applications outside of scientific research. The properties of dubnium, including its chemical behavior, are mostly inferred from its position in the periodic table, as it is expected to resemble the elements in group 5, particularly tantalum and niobium, in its chemical properties.
Dubnium (Db) is a synthetic, highly radioactive element with the atomic number 105. As an element, its formula is represented simply by its symbol, “Db,” and it consists of a single dubnium atom. In its elemental state, dubnium does not form bonds as it exists as a pure element, but it has the potential to form both covalent and ionic bonds when interacting with other elements. The chemical behavior of dubnium is not thoroughly studied due to its scarcity and short-lived nature.
Regarding its molecular structure, dubnium does not form conventional molecules as an isolated element. It is anticipated to exhibit the traits of a heavy, possibly volatile metal, with a crystalline structure that might resemble those of its group 5 counterparts, such as vanadium (V), niobium (Nb), and tantalum (Ta). However, any definitive description of its properties remains speculative.
Dubnium is expected to participate in electron sharing through covalent bonding or electron transfer in ionic interactions, based on predictions from its placement in the periodic table. Yet, the lack of experimental data limits a complete understanding of these interactions.
The significance of dubnium lies in its role as a superheavy element created in particle accelerators, which aids in the exploration of the periodic table’s boundaries and the stability of heavy nuclei. While dubnium mainly serves as a topic for scientific research, exploring the unknown realms of chemistry, any potential practical applications remain speculative due to its extreme scarcity and transient existence.
| Property | Value |
|---|---|
| Atomic Number | 105 |
| Atomic Mass | [268] |
| State at Room Temperature | Presumed to be solid |
| Melting Point | Unknown, predicted to be similar to that of Ta |
| Boiling Point | Unknown, predicted to be similar to that of Ta |
| Density | Unknown, but predicted based on group trends |
| Color | Unknown, likely metallic |
| Electronegativity | Unknown, predictions based on periodic trends |
| Atomic Radius | Estimated based on computational models |
Dubnium (Db), with the atomic number 105, is a synthetic, highly radioactive element belonging to group 5 of the periodic table. Its chemical properties have largely been extrapolated from theoretical studies and the behavior of lighter homologs like tantalum (Ta) and niobium (Nb), given the challenges in observing Dubnium directly due to its scarce production and short half-life.
| Property | Value |
|---|---|
| Half-lives | Varies by isotope; generally short-lived |
| Isotopes | Known isotopes range from Db-255 to Db-270 |
| Nuclear Spin | Theoretical prediction for specific isotopes |
| Decay Modes | Alpha decay predominantly; some isotopes may undergo spontaneous fission |
The preparation of Dubnium (Db), a synthetic element with atomic number 105, involves highly specialized nuclear reactions conducted in particle accelerators. Dubnium does not exist naturally due to its highly unstable and radioactive nature. The creation of Dubnium typically involves bombarding lighter elements with heavy ions to achieve nuclear fusion. Below is a summary of the processes used to synthesize Dubnium:
1.Dubnium Hexachloride (DbCl₆)
2.Dubnium Dioxide (DbO₂)
3.Dubnium Hydroxide (Db(OH)₄)
4.Dubnium Trifluoride (DbF₃)
5.Dubnium Nitrate (Db(NO₃)₄)
6.Dubnium Disulfide (DbS₂)
| Isotope | Mass Number | Half-life | Mode of Decay |
|---|---|---|---|
| Db-255 | 255 | 1.6 seconds | Alpha decay |
| Db-256 | 256 | 1.5 seconds | Alpha decay, Spontaneous fission |
| Db-257 | 257 | 2.2 seconds | Alpha decay |
| Db-258 | 258 | 4.5 seconds | Alpha decay, Spontaneous fission |
| Db-259 | 259 | 0.5 seconds | Alpha decay |
| Db-260 | 260 | 1.52 seconds | Alpha decay, Spontaneous fission |
| Db-261 | 261 | 1.8 seconds | Alpha decay |
| Db-262 | 262 | 34 seconds | Alpha decay, Spontaneous fission |
| Db-263 | 263 | 27 seconds | Alpha decay |
| Db-264 | 264 | 40 seconds | Spontaneous fission |
| Db-265 | 265 | 16 hours | Alpha decay |
| Db-266 | 266 | 1.2 hours | Alpha decay, Spontaneous fission |
| Db-267 | 267 | 3 hours | Alpha decay |
| Db-268 | 268 | 29 hours | Alpha decay |
| Db-270 | 270 | 23.15 hours | Alpha decay |
Dubnium (Db) is a synthetic element with no stable isotopes, making its practical applications outside of scientific research quite limited. The primary use of Dubnium, like many synthetic elements, lies in the field of scientific research, particularly in the areas of nuclear physics and chemistry. Here are some specific contexts in which Dubnium is utilized:
The production of Dubnium (Db), a synthetic and highly radioactive element, is achieved through nuclear reactions in particle accelerators. This process involves bombarding target atoms of lighter elements with ions of other elements at high energies. The methods and reactions used to produce Dubnium are complex, requiring sophisticated equipment and precise control. Here’s an overview of the primary methods used in the production of Dubnium:
Dubnium (Db), a synthetic and highly radioactive element with no stable isotopes, primarily finds its applications within the realm of scientific research rather than practical, everyday uses. Its incredibly short half-life and the complexity involved in its production mean that Dubnium’s applications are confined to theoretical and experimental domains. Here are some of the key applications of Dubnium:
Dubnium stands as a testament to human curiosity and scientific endeavor, offering insights into the complex nature of superheavy elements. Despite its limited practical applications, the study of Dubnium enriches our understanding of the periodic table, challenges existing theories of nuclear stability, and drives advances in nuclear physics and chemistry, underscoring the invaluable role of research in expanding human knowledge.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of dubnium?
104
105
106
107
Dubnium belongs to which group in the periodic table?
Group 4
Group 5
Group 6
Group 7
What is the symbol for dubnium?
Db
Dm
Du
Dbm
In what year was dubnium first synthesized?
1960
1965
1970
1975
What is the most stable isotope of dubnium?
Db-260
Db-262
Db-264
Db-268
What type of element is dubnium classified as?
Alkali metal
Lanthanide
Transition metal
Actinide
What is the primary method used to produce dubnium?
Electrolysis
Fusion of lighter nuclei
Chemical reduction
Distillation
What is the half-life of the isotope Db-262?
10 seconds
34 seconds
1 minute
10 minutes
Which of the following elements is chemically similar to dubnium?
Titanium
Chromium
Tantalum
Tungsten
Which of the following properties is true for dubnium?
High natural abundance
Radioactive
Low melting point
Non-metal
Before you leave, take our quick quiz to enhance your learning!

