What is the atomic number of Dysprosium?
64
65
66
67
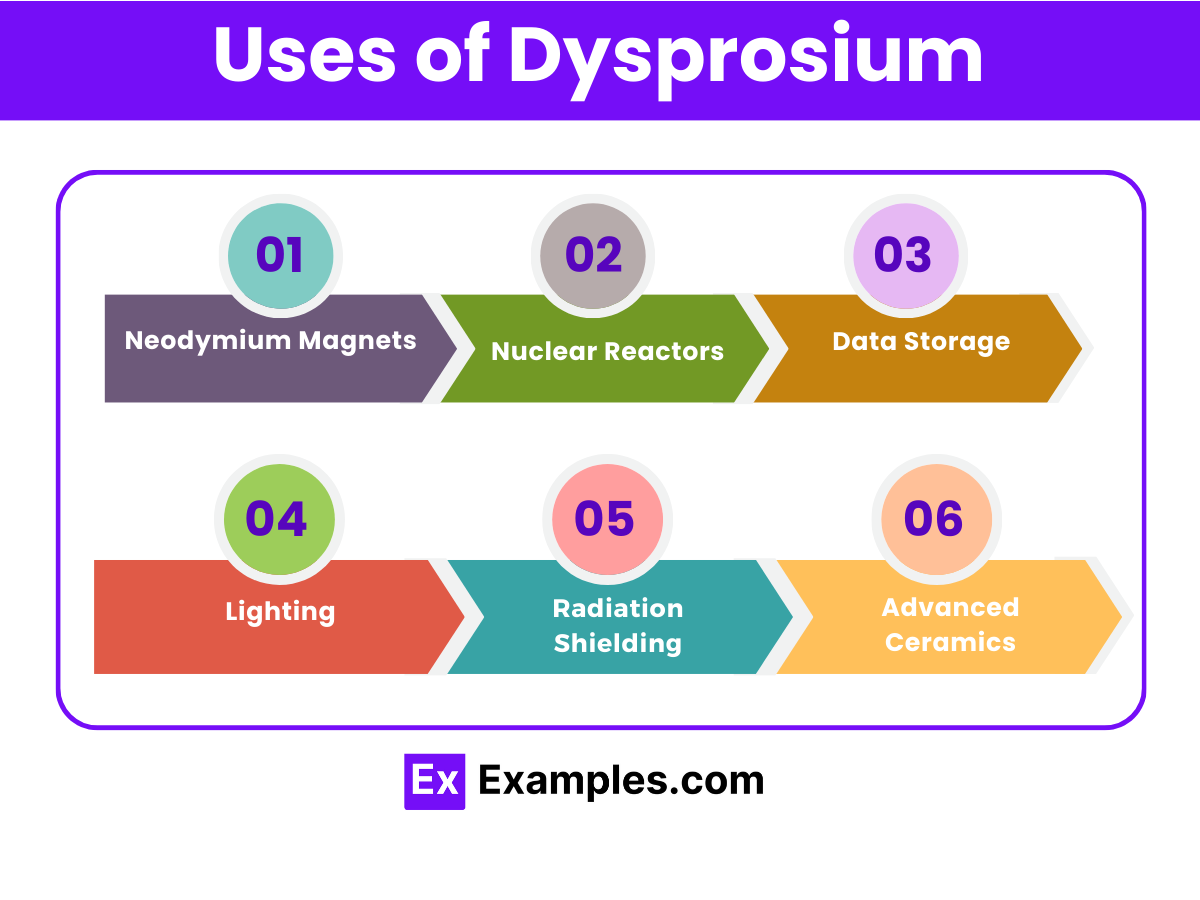
Discover the wonders of Dysprosium, a lesser-known yet incredibly valuable element in the periodic table. This complete guide illuminates Dysprosium’s unique properties, its pivotal role in modern technology, and its various applications. Through detailed examples, we’ll explore how Dysprosium enhances high-tech devices, contributes to green energy solutions, and stands out in scientific research. Dive into the world of Dysprosium to uncover its significance, uses, and the innovative compounds it forms, enriching our technological landscape.
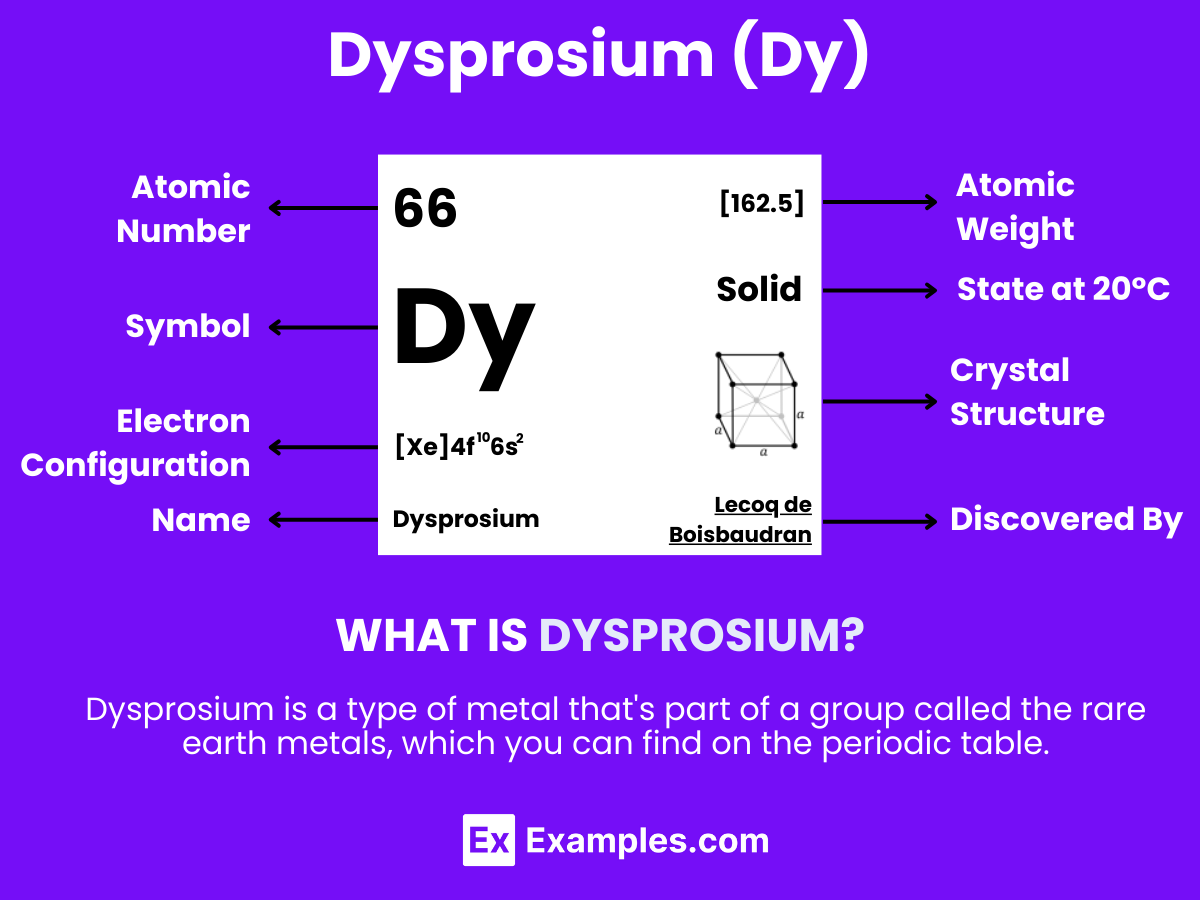
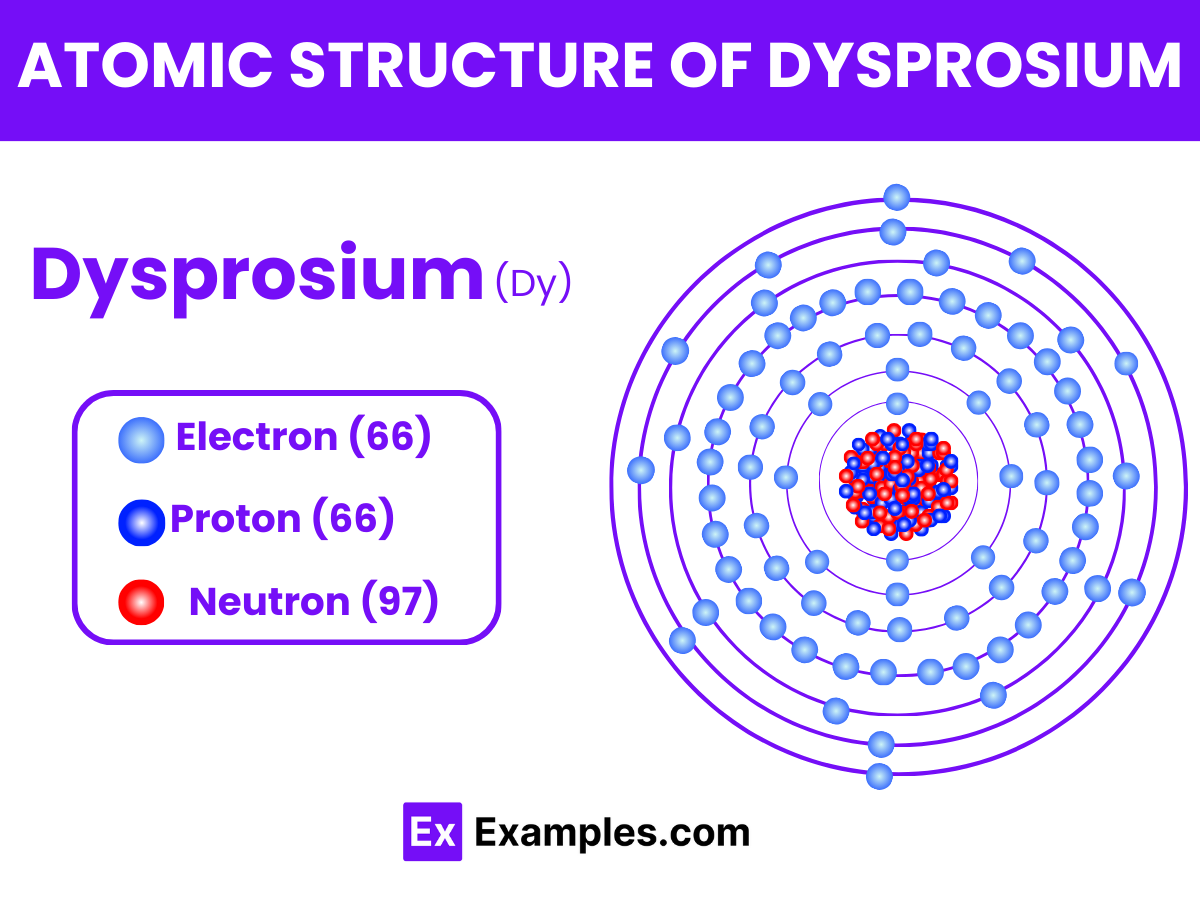
Dysprosium is a chemical element with the symbol Dy and atomic number 66. It is one of the lanthanides, a series of rare earth elements, and is known for its metallic and bright silver luster. Dysprosium has unique properties that make it highly valuable in various high-tech applications. Despite being called a “rare” earth element, dysprosium is relatively abundant in the Earth’s crust, comparable to some common metals like lead, but it is difficult to extract in pure form due to its presence in mixed rare earth minerals.
| Lanthanum | Gadolinium |
| Cerium | Terbium |
| Praseodymium | Lutetium |
| Neodymium | Holmium |
| Promethium | Erbium |
| Samarium | Thulium |
| Europium | Ytterbium |
| Physical Property | Value |
|---|---|
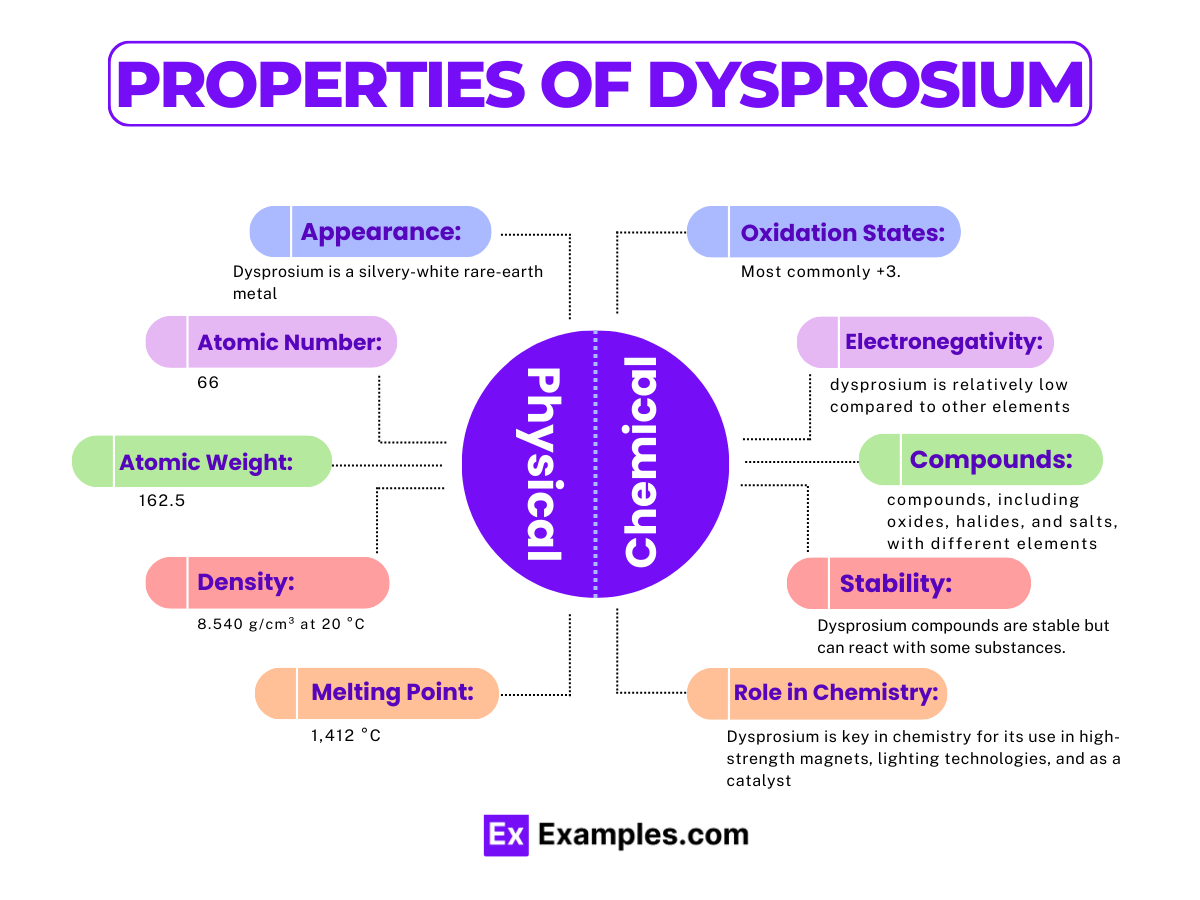
| Appearance | Silvery white, metallic |
| Melting Point | 1,412 °C (2,574 °F) |
| Boiling Point | 2,567 °C (4,653 °F) |
| Density | 8.540 g/cm³ at 20 °C |
| Phase at Room Temperature | Solid |
| Thermal Conductivity | 10.7 W/(m·K) |
| Electrical Resistivity | 926 nΩ·m at 20 °C |
| Magnetic Ordering | Paramagnetic |
Dysprosium (Dy) is a rare earth element known for its large magnetic susceptibility. It belongs to the lanthanide series in the periodic table and exhibits interesting chemical properties due to its electron configuration. Here, we delve into the chemical properties of dysprosium, emphasizing its reactions, common oxidation states, and examples of its compounds.
Oxidation States: The most common oxidation state of dysprosium is +3 Dy³⁺. This oxidation state is prevalent in almost all dysprosium compounds, reflecting its stable electron configuration [Xe] 4f¹⁰ 6s².
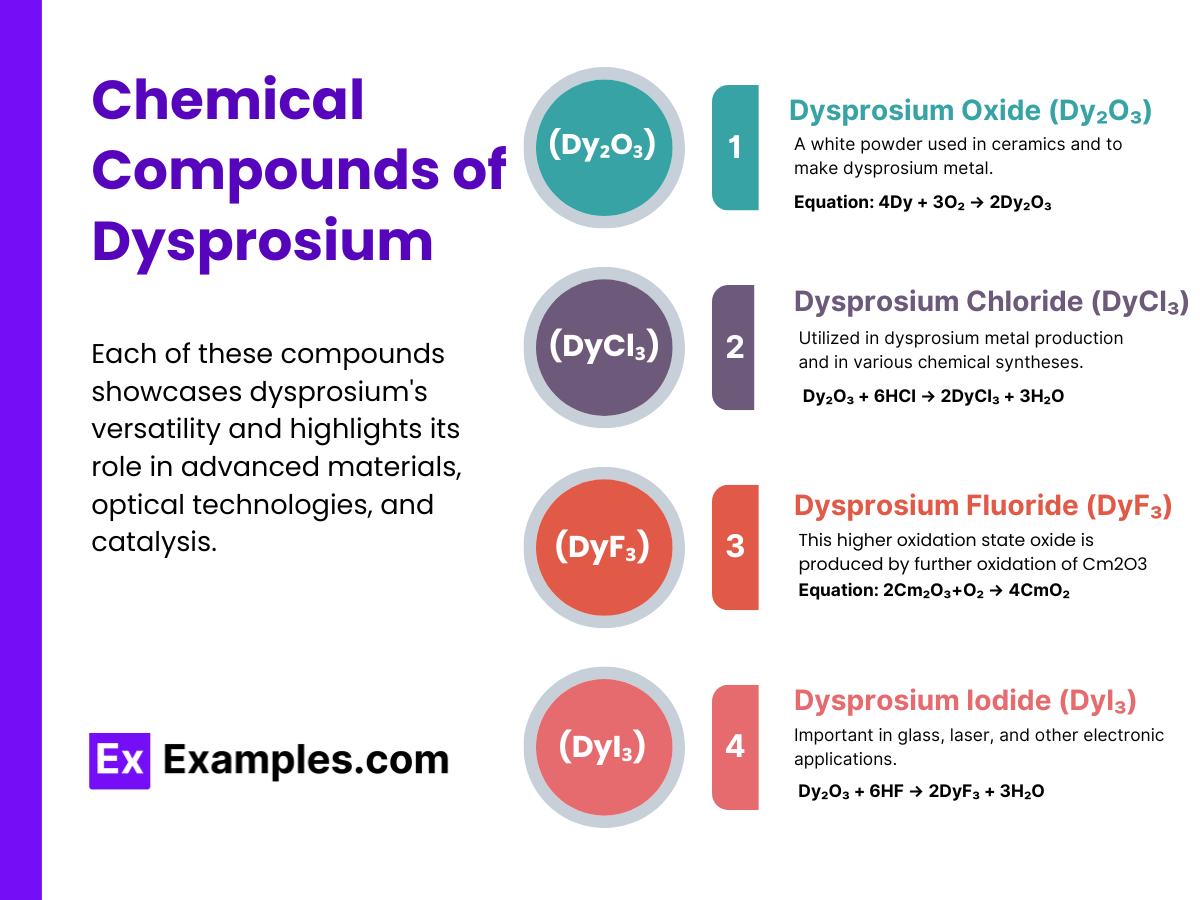
Reactivity Dysprosium is relatively stable in dry air but oxidizes in moist air, forming dysprosium(III) oxide:
3Dy + O₂ → 2Dy₂O₃
This reaction demonstrates dysprosium’s reactivity towards oxygen, leading to the formation of a white oxide layer on the metal’s surface.
Reaction with Water Dysprosium reacts with water, albeit slowly, to form dysprosium hydroxide and hydrogen gas:
2 Dy+6 H2O→2 Dy(OH)₃+3 H₂
| Thermodynamic Property | Value |
|---|---|
| Melting Point | 1,412 °C |
| Boiling Point | 2,567 °C |
| Standard Molar Entropy (S°) | 192.0 J/(mol·K) |
| Standard Enthalpy of Formation (ΔHf°) | -185 kJ/mol (for Dy2O3) |
| Material Property | Value |
|---|---|
| Crystal Structure | Hexagonal close-packed (hcp) |
| Hardness | Relatively soft, malleable |
| Modulus of Elasticity | 61.4 GPa |
| Thermal Expansion | 9.9 µm/(m·K) at 25 °C |
| Electromagnetic Property | Value |
|---|---|
| Electrical Resistivity | 926 nΩ·m at 20 °C |
| Magnetic Ordering | Paramagnetic at 300 K |
| Curie Temperature | Approximately 88 K |
| Nuclear Property | Value |
|---|---|
| Natural Isotopes | Dy-156, Dy-158, Dy-160, Dy-161, Dy-162, Dy-163, Dy-164 |
| Most Stable Isotope | Dy-164 with a half-life of over 1.8×10141.8×1014 years |
| Neutron Cross Section | 994 barns (for Dy-164) |
| Isotopic Abundance | Dy-164: 28.18%, Dy-162: 25.51%, etc. |
The preparation of dysprosium typically involves the extraction and purification processes from minerals like monazite and bastnäsite, which contain dysprosium in the form of oxides. The process begins with the crushing of these minerals, followed by acid or alkali treatments to produce a mixture of rare earth chlorides. Dysprosium is then separated from other rare earths through solvent extraction or ion exchange techniques. Finally, the dysprosium chloride is reduced with metallic calcium in a high-temperature vacuum environment to produce pure dysprosium metal: DyCl₃ + 3Ca → Dy + 3CaCl₂
The production of dysprosium involves complex processes, primarily starting from the mining of rare earth-containing minerals like monazite and bastnäsite. The extraction process includes crushing the ore, acid leaching to obtain mixed rare earth chlorides or oxides, and then separating dysprosium from other rare earths using solvent extraction or ion-exchange methods. The final step involves reducing dysprosium fluoride with calcium metal in an inert atmosphere to produce pure dysprosium metal.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Dysprosium?
64
65
66
67
Dysprosium belongs to which group of elements?
Alkaline earth metals
Transition metals
Lanthanides
Halogens
What is the primary use of Dysprosium in modern technology?
Construction materials
Magnets in electric motors
Fertilizers
Jewelry making
What is the symbol of Dysprosium in the periodic table?
Dy
Dp
Ds
Do
In which type of reactors is Dysprosium used to control nuclear fission?
Fusion reactors
Breeder reactors
Fast-neutron reactors
Nuclear fission reactors
What is the primary oxidation state of Dysprosium?
+1
+2
+3
+4
What is the natural state of Dysprosium at room temperature?
Gas
Liquid
Solid
Plasma
Which property of Dysprosium makes it useful in nuclear control rods?
High electrical conductivity
High neutron absorption
Low melting point
Low thermal conductivity
Dysprosium is often alloyed with which other element to improve its magnetic properties?
Iron
Copper
Zinc
Gold
In which year was Dysprosium discovered?
1867
1886
1899
1903
Before you leave, take our quick quiz to enhance your learning!

