What is the chemical formula for ethanol?
CH3CH2OH
C2H5OH
C2H6O
CH3OH
Ethanol is commonly known as alcohol and it is a clear and colorless liquid that holds a significant place in the world of chemistry. It is made through the fermentation of sugars by yeast or via a chemical process involving ethylene. At its core, ethanol is a covalent compound, meaning its atoms share electrons in bonds to form molecules. This sharing is a fundamental concept in chemistry, illustrating how elements come together to create substances with unique properties. Ethanol is not only essential in scientific research but also widely used in everyday life, from being a key ingredient in alcoholic beverages to serving as a clean-burning fuel additive.
Ethanol, also known as ethyl alcohol or simply alcohol, is a chemical compound that is both colorless and flammable. It has the formula C₂H₅OH, featuring a two-carbon chain with an alcohol group attached, making it a member of the alcohol family of compounds. Ethanol is most famous for being the type of alcohol found in alcoholic beverages, but it also has many other uses. It’s used as a solvent in the medical and cosmetic industries, as a clean-burning fuel or fuel additive in the automotive sector, and in the manufacture of perfumes and varnishes. Its versatility makes it an important substance in both daily life and various industrial processes.
| Property | Value |
|---|---|
| Formula | C₂H₅OH |
| Hill Formula | C₂H₆O |
| Name | Ethanol |
| Alternate Names | Algrain; Anhydrol, Fermentation Alcohol, Grain Alcohol, Potato Alcohol, Tecsol |
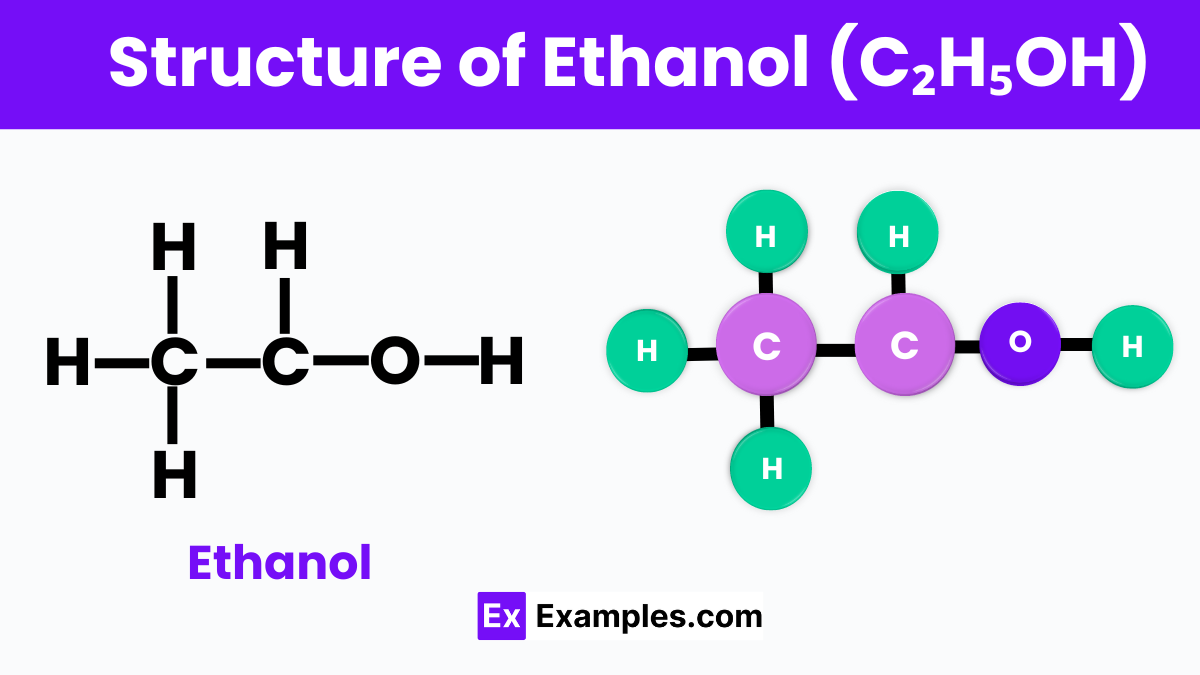
Ethanol, often represented by the chemical formula C₂H₅OH. At its core, ethanol is made up of two carbon atoms, six hydrogen atoms, and one oxygen atom. These atoms are arranged in a way that the two carbon atoms form a chain, with one end of the chain attaching to an oxygen atom. This oxygen atom is also connected to a hydrogen atom, forming what we call an alcohol group (-OH). This specific arrangement is what makes ethanol an alcohol.
The bond between the oxygen and the hydrogen atom in the alcohol group is particularly important because it gives ethanol its characteristic properties, such as its ability to mix well with water and other organic compounds. This versatility makes ethanol invaluable in everyday products like alcoholic beverages, sanitizers, and fuels. Understanding the structure of ethanol helps us appreciate not just its chemical identity but also its widespread utility in our lives.
Ethanol can be made through two primary methods: fermentation and hydration of ethylene. Fermentation is the more traditional method, where sugars found in fruits or grains are converted into ethanol and carbon dioxide by the action of yeast. The basic equation for fermentation is:
Another common method, especially in industrial settings, is the hydration of ethylene. In this chemical reaction, ethylene gas reacts with water, usually in the presence of an acid catalyst like phosphoric acid, to produce ethanol. The equation for this process is:
Both methods are widely used to produce ethanol, each having its own advantages depending on the desired scale and purity of the ethanol produced. Fermentation is key for producing alcoholic beverages, while hydration of ethylene is crucial for creating ethanol for fuel and industrial uses.
| Property | Description |
|---|---|
| Appearance | Clear, colorless liquid |
| Odor | Characteristic, alcoholic |
| Boiling Point | 78.37°C (173°F) |
| Melting Point | -114.1°C (-173.4°F) |
| Density | 0.789 g/cm³ (at 20°C) |
| Solubility | Miscible with water and most organic solvents |
| Molecular Weight | 46.07 g/mol |
| Viscosity | Slightly more viscous than water |
When ethanol reacts with metallic sodium, it produces sodium ethoxide and hydrogen gas. This reaction demonstrates ethanol’s ability to act as an acid.
Equation: 2C₂H₅OH + 2Na → 2C₂H₅ONa + H₂
Ethanol burns in the presence of oxygen to produce carbon dioxide, water, and heat. This combustion reaction is what makes ethanol a valuable fuel.
Equation: C₂H₅OH + 3O₂ → 2CO₂ + 3H₂O + heat
When heated with a catalyst, ethanol can be dehydrated to form either ethene (ethylene) or diethyl ether, depending on the conditions. Dehydration to ethene is a common reaction used in the production of various chemicals.
Equation: C₂H₅OH → H₂O + C₂H₄ (in the presence of heat and catalyst)
Oxidation
Ethanol can be oxidized to produce acetaldehyde and further to acetic acid, depending on the conditions and the oxidizing agent used. This property is significant in biochemical processes and industrial chemistry.
Equation: C₂H₅OH + O₂ → CH₃CHO + H₂O (to acetaldehyde)
CH₃CHO + O₂ → CH₃COOH (to acetic acid)
Ethanol reacts with carboxylic acids in the presence of an acid catalyst to form esters and water. This reaction is used in the production of various esters, which are important in manufacturing flavors and fragrances.
Equation: C₂H₅OH + RCOOH → RCOOC₂H₅ + H₂O
| Property | Value |
|---|---|
| CAS Registry Number | 64-17-5 |
| Beilstein Number | 1718733 |
| PubChem Compound ID | 702 |
| PubChem Substance ID | 24851272 |
| SMILES Identifier | CCO |
| InChI Identifier | InChI=1/C2H6O/c1-2-3/h3H, 2H2, 1H3 |
| InChI Key | LFQSCWFLJHTTHZ-UHFFFAOYAB |
| MDL Number | MFCD00003568 |
| Property | Value |
|---|---|
| NFPA Health Rating | 0 |
| NFPA Fire Rating | 3 |
| NFPA Reactivity Rating | 0 |
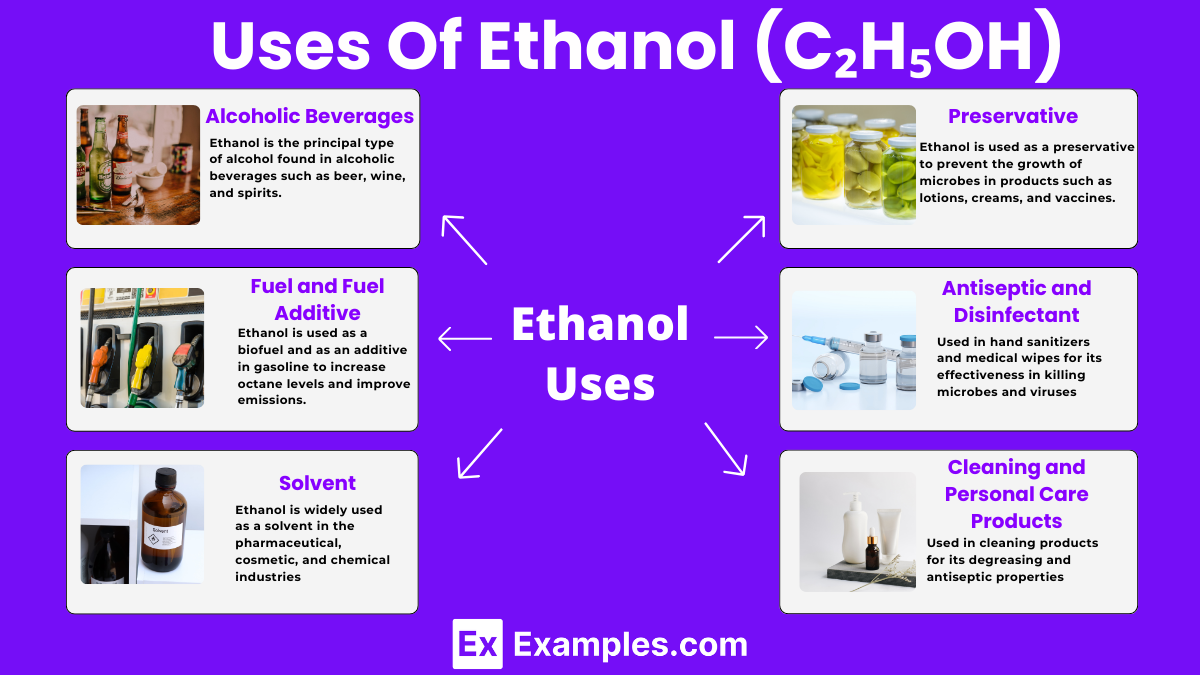
Ethanol is the principal type of alcohol found in alcoholic beverages such as beer, wine, and spirits. It is produced through the fermentation of sugars by yeast.
Ethanol is used as a biofuel and as an additive in gasoline to increase octane levels and improve emissions. It helps reduce carbon monoxide and other harmful emissions from vehicles.
Due to its ability to dissolve both polar and non-polar substances, ethanol is widely used as a solvent in the pharmaceutical, cosmetic, and chemical industries. It is ideal for crafting perfumes, varnishes, and medicines.
Ethanol has disinfectant properties and is used in hand sanitizers and medical wipes for its effectiveness in killing microbes and viruses, helping to prevent the spread of infections.
In the pharmaceutical and cosmetics industries, ethanol is used as a preservative to prevent the growth of microbes in products such as lotions, creams, and vaccines.
Ethanol serves as a key ingredient in the production of various chemicals, including ethyl esters, acetic acid, and other important industrial chemicals. Its reactivity makes it a valuable starting material in chemical synthesis.
Ethanol is used in cleaning products for its degreasing and antiseptic properties. It is also found in personal care products like perfumes and aftershaves for its solvent properties and quick evaporation.
Ethanol, made from plants like corn and sugarcane, is a renewable and more sustainable energy source compared to fossil fuels.
Using ethanol in place of traditional fuels can lead to lower emissions of harmful pollutants, helping to fight climate change.
Ethanol is environmentally friendly as it breaks down quickly and naturally, reducing the risk of pollution.
Its ability to dissolve various substances makes ethanol crucial in making many pharmaceuticals and cosmetics.
Ethanol is effective in killing bacteria and viruses, making it a key ingredient in sanitizers and disinfectants.
Ethanol production provides a significant market for agricultural products, benefiting farmers and rural economies.
Domestic ethanol production reduces reliance on imported oil, enhancing a country’s energy independence.
Yes, ethanol is a type of alcohol, specifically the one safe for consumption in beverages, also known as ethyl alcohol.
Pure ethanol is 100% alcohol by volume, but commercial ethanol is often mixed with water to make it less potent.
Yes, ethanol is used as a renewable fuel and fuel additive, known for reducing emissions and enhancing octane levels.
Some cars, especially flex-fuel vehicles, can run on 100% ethanol, though they are more common in countries producing large amounts of ethanol.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical formula for ethanol?
CH3CH2OH
C2H5OH
C2H6O
CH3OH
Ethanol is primarily used as:
A preservative
A solvent
A fuel additive
All of the above
Which process is commonly used to produce ethanol from corn?
Hydrolysis
Fermentation
Distillation
Polymerization
What is the main environmental benefit of using ethanol as a fuel?
It is cheaper than gasoline
It produces no CO2
It is renewable
It increases engine efficiency
Which type of alcohol is ethanol?
Primary alcohol
Secondary alcohol
Tertiary alcohol
Quaternary alcohol
The intoxicating effect of alcoholic beverages is mainly due to which component?
Methanol
Ethanol
Propanol
Butanol
What hazard is associated with the inhalation of ethanol vapors?
Lung cancer
Respiratory irritation
Cognitive enhancement
Skin hydration
In addition to drinking, what is a common use of ethanol in healthcare?
As a diuretic
As an antiseptic
As a sedative
As an analgesic
Which characteristic of ethanol allows it to mix well with water and organic compounds?
Volatility
Polarity
Non-polarity
Reactivity
What by-product is formed when ethanol is burned in an engine?
Water
Oxygen
Carbon monoxide
Nitrogen oxides
Before you leave, take our quick quiz to enhance your learning!

