What is the chemical symbol for Fermium?
Fm
Fr
Fe
Fn
Dive into the captivating world of Fermium, an element that lies at the heart of nuclear chemistry and physics. This comprehensive guide unfolds the secrets of Fermium, from its discovery in the aftermath of atomic testing to its intricate production processes and the cutting-edge research it enables. With detailed examples, we highlight Fermium’s role in expanding our understanding of the atomic nucleus, its applications in synthesizing new elements, and its contributions to scientific advancements. Embark on a journey to explore the intriguing properties and potential uses of Fermium, a testament to human curiosity and the relentless pursuit of knowledge in the atomic age.
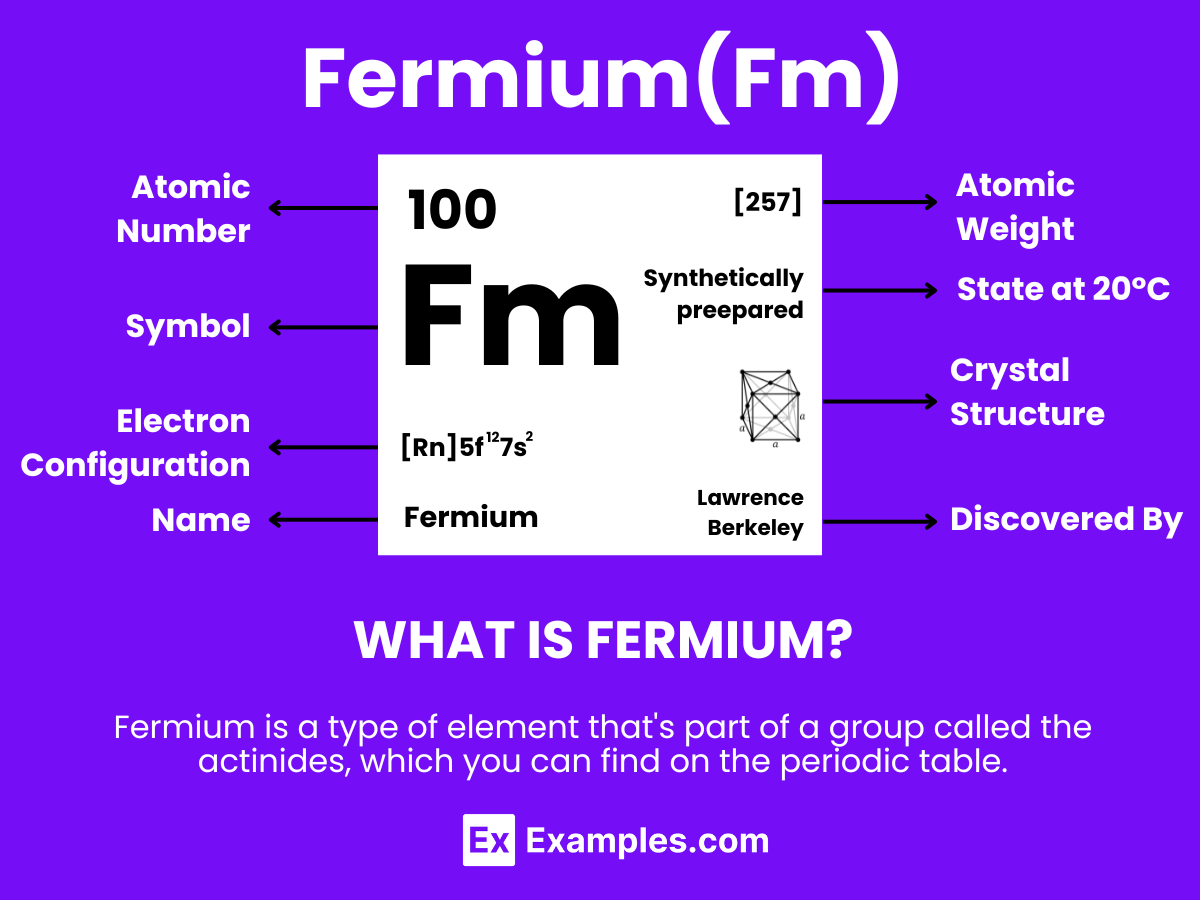
Fermium is a highly radioactive, synthetic element with the atomic number 100. It is a member of the actinide series, characterized by its metallic properties under certain conditions. Fermium does not occur naturally but is produced artificially in very small amounts through the bombardment of lighter elements with neutrons or in the debris of nuclear explosions. This element is known for its high level of radioactivity and is primarily used for scientific research rather than commercial applications. Due to its radioactive nature, Fermium requires specialized handling and containment facilities.
| Actinium | Berkelium |
| Thorium | Californium |
| Protactinium | Einsteinium |
| Uranium | Curium |
| Neptunium | Mendelevium |
| Plutonium | Nobelium |
| Americium | Lawrencium |
1. Atomic Number and Symbol
2. Electron Configuration
3. Isotopes
4. Atomic Mass
5. Physical Properties
6. Chemical Properties
7. Role in Research
8. Applications
| Property | Value |
|---|---|
| Appearance | Metallic, with a silvery luster |
| Phase at Room Temperature | Solid |
| Density | Estimated to be about 9.7 g/cm³ (predicted for Fm-257) |
| Melting Point | Approx. 1527°C (estimated) |
| Boiling Point | Unknown |
| Atomic Mass | Various isotopes; Fm-257 is the most stable with an atomic mass of 257 u |
| Crystal Structure | Face-centered cubic (fcc), predicted |
| State of Matter | Radioactive metal |
Fermium, a synthetic and highly radioactive element in the actinide series, exhibits several chemical properties that are noteworthy:
| Property | Description |
|---|---|
| Melting Point | Estimated: 1527 °C (Estimate; may vary) |
| Boiling Point | Unknown; Predicted to be around 1000 °C (Estimate) |
| Heat of Fusion | Data not available |
| Heat of Vaporization | Data not available |
| Specific Heat Capacity | Data not available |
| Thermal Conductivity | Data not available |
| Property | Description |
|---|---|
| State at Room Temperature | Solid (Presumed) |
| Density | Estimated: ~9.7 g/cm³ at 20 °C (Estimate) |
| Crystal Structure | Unknown; Predicted to be Face-centered Cubic (FCC) |
| Hardness | Data not available |
| Malleability | Data not available |
| Ductility | Data not available |
| Property | Description |
|---|---|
| Electrical Conductivity | Data not available |
| Magnetic Ordering | Data not available |
| Superconductivity | Not observed |
| Electronegativity | Data not available |
| Ionization Energies | First: Data not available |
| Property | Description |
|---|---|
| Atomic Number | 100 |
| Atomic Mass | Isotopes range from Fermium-242 to Fermium-257 |
| Half-lives | Varies; Fermium-257 has a half-life of about 100.5 days |
| Decay Modes | Alpha decay, Spontaneous fission |
| Neutron Cross Section | Data not available |
| Neutron Mass Absorption | Data not available |
Introduction to Fermium
Discovery of Fermium
Production Methods
Nuclear Reactions
Particle Accelerators
Isolation of Fermium
Characterization of Fermium
Applications of Fermium
Safety and Handling
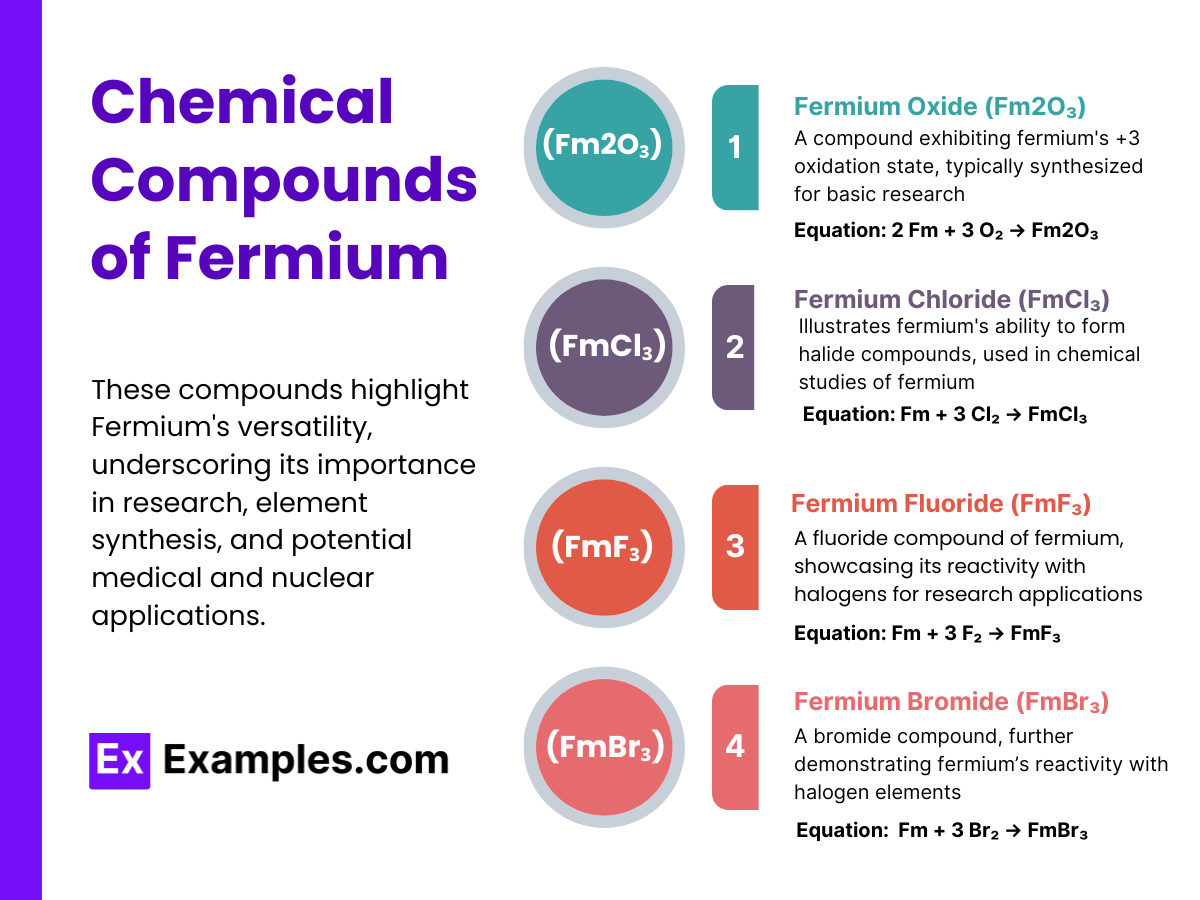
Fermium, a synthetic element with the symbol Fm and atomic number 100, does not occur naturally and must be synthesized in a laboratory. It has several isotopes, each with a different number of neutrons. Below is a table outlining some of the known isotopes of fermium:
| Isotope | Mass Number | Half-life | Decay Mode |
|---|---|---|---|
| Fm-252 | 252 | 25.39 hours | Alpha decay |
| Fm-253 | 253 | 3 days | Alpha decay |
| Fm-254 | 254 | 3.24 hours | Alpha decay, Spontaneous fission |
| Fm-255 | 255 | 20.07 hours | Alpha decay |
| Fm-256 | 256 | 2.63 hours | Spontaneous fission, Alpha decay |
| Fm-257 | 257 | 100.5 days | Alpha decay |
Due to its high radioactivity and the difficulty in producing significant quantities, fermium does not have many practical applications outside of scientific research. The primary uses and studies of fermium involve:
This article underscores the complex journey of Fermium’s discovery, synthesis, and investigation. Despite its scarcity and radioactivity, Fermium fascinates scientists, offering insights into nuclear chemistry and the actinides’ behaviors. As research advances, our understanding of Fermium will deepen, potentially unveiling new aspects of atomic science and material properties
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical symbol for Fermium?
Fm
Fr
Fe
Fn
What is the atomic number of Fermium?
100
101
102
103
In which year was Fermium discovered?
1939
1944
1952
1960
Who were the scientists that discovered Fermium?
Marie Curie and Pierre Curie
Albert Einstein and Enrico Fermi
Albert Ghiorso, Ralph A. James, and Albert A. Michelson
Ernest Rutherford and Niels Bohr
What is the classification of Fermium on the periodic table?
Lanthanide
Actinide
Transition metal
Noble gas
What type of element is Fermium in terms of its radioactivity?
Stable
Non-radioactive
Radioactive
Rare
What is the most common isotope of Fermium?
Fermium-100
Fermium-102
Fermium-257
Fermium-256
Which of the following is a known application of Fermium?
Medical imaging
Nuclear reactors
Nuclear weapons
Food preservation
What is the most significant challenge associated with Fermium?
It is highly stable
It is not radioactive
It is extremely rare and difficult to produce
It is inexpensive to produce
Which group in the periodic table does Fermium belong to?
Alkaline earth metals
Halogens
Noble gases
Actinides
Before you leave, take our quick quiz to enhance your learning!

