What is the atomic number of Flerovium?
112
114
116
118
Dive into the fascinating realm of superheavy elements with our comprehensive guide on Flerovium. This elusive element, known for its position in the periodic table, is shrouded in mystery and scientific intrigue. Flerovium stands at the forefront of nuclear research, offering insights into the synthesis and behavior of superheavy elements. Through detailed examples, we’ll explore Flerovium’s discovery, unique properties, potential uses, and the groundbreaking compounds it forms, enriching your knowledge on this cutting-edge scientific frontier.
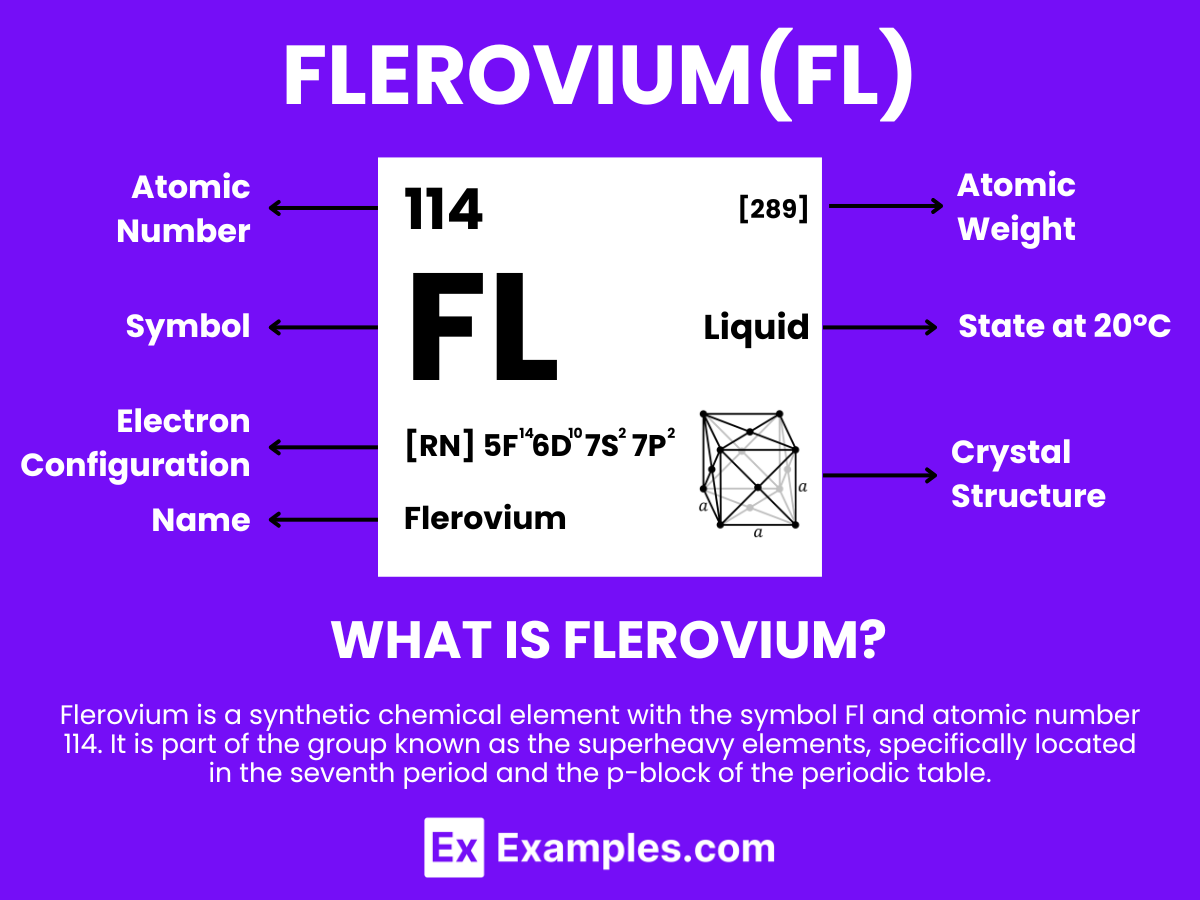
Flerovium is a synthetic chemical element with the symbol Fl and atomic number 114. It is part of the group known as the superheavy elements, specifically located in the seventh period and the p-block of the periodic table. Flerovium is named after the Flerov Laboratory of Nuclear Reactions of the Joint Institute for Nuclear Research in Dubna, Russia, where the element was first synthesized in 1998 by a Russian-American collaboration team.As a superheavy element, flerovium does not occur naturally and is produced in a laboratory through the fusion of lighter nuclei, such as bombarding plutonium targets with calcium ions. Its most stable known isotopes have very short half-lives, on the order of a few seconds, making it challenging to study. Due to its short-lived existence, research on flerovium is primarily focused on understanding its nuclear properties and its position in the periodic table, rather than on practical applications.
[/ns_callout]
| Meitnerium | Darmstadtium | Roentgenium |
| Copernicium | Nihonium | Moscovium |
| Livermorium | Tennessine | Oganesson |
Formula: Fl
Composition: Consists of a single flerovium atom.
Bond Type: In its elemental form, flerovium does not have bonds as it is a pure element. However, flerovium can form covalent or ionic bonds when reacting with other elements, although its chemical reactivity is not well-studied due to its rarity and short half-life.
Molecular Structure: As a pure element, flerovium does not form a molecular structure in the same sense as compounds. It is predicted to be a heavy, volatile metal with a possible close-packed crystalline structure, though its exact physical and chemical properties remain largely speculative.
Electron Sharing: In compounds, flerovium is expected to share electrons covalently or transfer electrons ionically, depending on the nature of the other element(s) it is bonding with. Its chemistry is predicted based on its position in the periodic table, but experimental data is limited.
Significance: Flerovium is notable for being a superheavy element synthesized in particle accelerators. Its study helps scientists understand the properties of elements at the edge of the periodic table and the stability of superheavy nuclei.
Role in Chemistry: Flerovium’s role in chemistry is primarily in the field of research, particularly in studies aimed at exploring the limits of the periodic table and the synthesis of new elements. Its potential applications outside of scientific research are currently unknown due to its extreme rarity and instability.
Flerovium (Fl) is a superheavy synthetic element with the atomic number 114 on the periodic table. Named after the Flerov Laboratory of Nuclear Reactions where it was first synthesized, Flerovium’s atomic structure exhibits some fascinating characteristics that distinguish it from lighter elements. Understanding its atomic structure requires delving into its electronic configuration, nuclear composition, and predicted chemical properties, which are all influenced by its position in the periodic table and the effects of relativistic mechanics on its electrons.
Flerovium’s electronic configuration is theorized to be [Rn] 5f¹⁴ 6d¹⁰ 7s² 7p² placing it in the p-block of the periodic table. This configuration suggests that Flerovium may exhibit some properties similar to lead (Pb), which is directly above it in Group 14. However, due to relativistic effects, the actual chemistry of Flerovium may deviate significantly from these predictions.
The nucleus of Flerovium atoms contains 114 protons, which defines its position as the 114th element in the periodic table. The number of neutrons in Flerovium can vary, leading to the creation of different isotopes. The most stable isotope known to date is Flerovium-289, with 175 neutrons. These isotopes are characterized by extremely short half-lives, with Flerovium-289 having a half-life of about 2.6 seconds, indicating the inherent instability of superheavy elements.
The atomic structure of Flerovium is significantly influenced by relativistic effects. As electrons in heavy elements move at speeds approaching a significant fraction of the speed of light, their mass increases, which in turn affects their interaction with the nucleus and with each other. These effects are particularly pronounced in superheavy elements like Flerovium, leading to changes in electron orbital shapes, energy levels, and chemical reactivity.
Based on its electronic configuration and the anticipated impact of relativistic effects, Flerovium is expected to have some unique properties:
| Property | Value |
|---|---|
| Atomic Number | 114 |
| Relative Atomic Mass | (289) |
| Density | Predicted to be high, potentially similar to or greater than lead |
| Melting Point | Unknown, but predicted to be low for a metal |
| Boiling Point | Unknown |
| State at Room Temperature | Presumed to be solid |
| Color | Unknown, potentially metallic |
| Half-life of Most Stable Isotope (Fl-289) | ~2.6 seconds |
Flerovium (Fl), with atomic number 114, resides in the p-block of the periodic table. Its chemical properties are inferred from theoretical predictions and relativistic calculations due to its extremely short half-life and the complexities associated with its production.
| Property | Value |
|---|---|
| Melting Point | Predicted to be low, possibly around the melting point of Lead or slightly higher |
| Boiling Point | Theoretical; expected to be similar to or slightly higher than that of Lead due to relativistic effects |
| Density | Predicted to be significantly high, potentially higher than Lead due to relativistic effects |
| Property | Value |
|---|---|
| State at STP | Predicted to possibly be a solid at room temperature |
| Crystal Structure | Unknown; theoretical predictions suggest a structure that could be influenced by strong relativistic effects |
| Thermal Conductivity | Expected to be low, in line with heavy metals and influenced by its electronic structure |
| Property | Value |
|---|---|
| Electrical Conductivity | Expected to be relatively poor, influenced by its electronic configuration |
| Electronegativity | Predicted based on its position; however, exact values are unknown due to lack of experimental data |
| Property | Value |
|---|---|
| Half-life of Most Stable Isotope (Fl-289) | About 2.6 seconds, showcasing the element’s instability |
| Decay Modes | Predominantly alpha decay; some isotopes may undergo spontaneous fission |
| Atomic Mass | Isotopes range observed: 284-289 amu, with a focus on nuclear stability and decay patterns |
The preparation of Flerovium, a superheavy synthetic element with the atomic number 114, involves highly sophisticated nuclear reactions conducted in particle accelerators. Flerovium does not occur naturally and can only be created artificially through the collision of lighter nuclei. The process to prepare Flerovium primarily involves bombarding a target material with accelerated ions of another element.
| Isotope | Half-Life | Decay Mode |
|---|---|---|
| Flerovium-284 | 2.5 seconds | Alpha decay to Copernicium-280 |
| Flerovium-285 | 0.89 milliseconds | Alpha decay to Copernicium-281 |
| Flerovium-286 | 0.13 seconds | Alpha decay to Copernicium-282 |
| Flerovium-287 | 0.48 seconds | Alpha decay to Copernicium-283 |
| Flerovium-288 | 0.8 seconds | Alpha decay to Copernicium-284 |
| Flerovium-289 | 1.9 seconds | Alpha decay to Copernicium-285 |
| Flerovium-290 | 19 seconds | Alpha decay to Copernicium-286 |
| Flerovium-291 | Unknown | Predicted alpha decay to Copernicium-287 |
Currently, Flerovium (Fl) does not have any practical uses outside of scientific research due to its extremely short half-life and the difficulty in producing it in significant quantities. Flerovium is a superheavy synthetic element that was first discovered in 1998, and its properties are not fully understood due to its instability and rarity. Here are the primary areas where Flerovium is of interest:
Flerovium, a superheavy synthetic element, is produced in highly specialized nuclear research facilities rather than being a byproduct of nuclear reactors like Technetium. Here’s an overview of its production process:
Currently, Flerovium (element 114) does not have practical applications due to its extremely short half-life and the challenges associated with producing it in sufficient quantities for practical use. As one of the superheavy elements, its existence is primarily of interest to scientific research aimed at understanding the properties of elements at the extreme end of the periodic table. Here are the potential areas of interest related to Flerovium:
Flerovium represents a frontier in the exploration of superheavy elements, offering insights into the limits of the periodic table and nuclear stability. While practical applications are yet to be realized due to its short half-life and production challenges, ongoing research on Flerovium could pave the way for future scientific breakthroughs and a deeper understanding of elemental behavior.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Flerovium?
112
114
116
118
Flerovium is part of which group in the periodic table?
Group 12
Group 13
Group 14
Group 15
Flerovium was named after which scientist?
Dmitri Mendeleev
Georgy Flyorov
Marie Curie
Niels Bohr
In which year was Flerovium first synthesized?
1998
2000
2004
2012
What is the symbol for Flerovium on the periodic table?
Fl
Fv
Ff
Fm
Which element is used to bombard plutonium to produce Flerovium?
Carbon
Calcium
Nitrogen
Oxygen
Flerovium is classified as which type of element?
Metal
Non-metal
Metalloid
Noble gas
What is the most stable isotope of Flerovium?
Fl-286
Fl-289
Fl-290
Fl-293
Flerovium is part of which period in the periodic table?
Period 6
Period 7
Period 8
Period 9
Which laboratory is credited with the discovery of Flerovium?
Lawrence Berkeley National Laboratory
Joint Institute for Nuclear Research
Oak Ridge National Laboratory
CERN
Before you leave, take our quick quiz to enhance your learning!

