What is the atomic number of Holmium?
64
65
66
67
The fascinating world of Holmium, an element that’s as mysterious as it is essential in the modern scientific landscape. This comprehensive guide shines a light on Holmium, from its core definition and meaning to the wide array of its applications and intriguing compounds. Delve into the uses of Holmium in technology and medicine, uncovering how this rare earth element plays a pivotal role in advancements that touch our daily lives. Rich in keywords and optimized for SEO and NLP, this introduction paves the way for an enlightening exploration of Holmium’s significant contributions to science and industry.
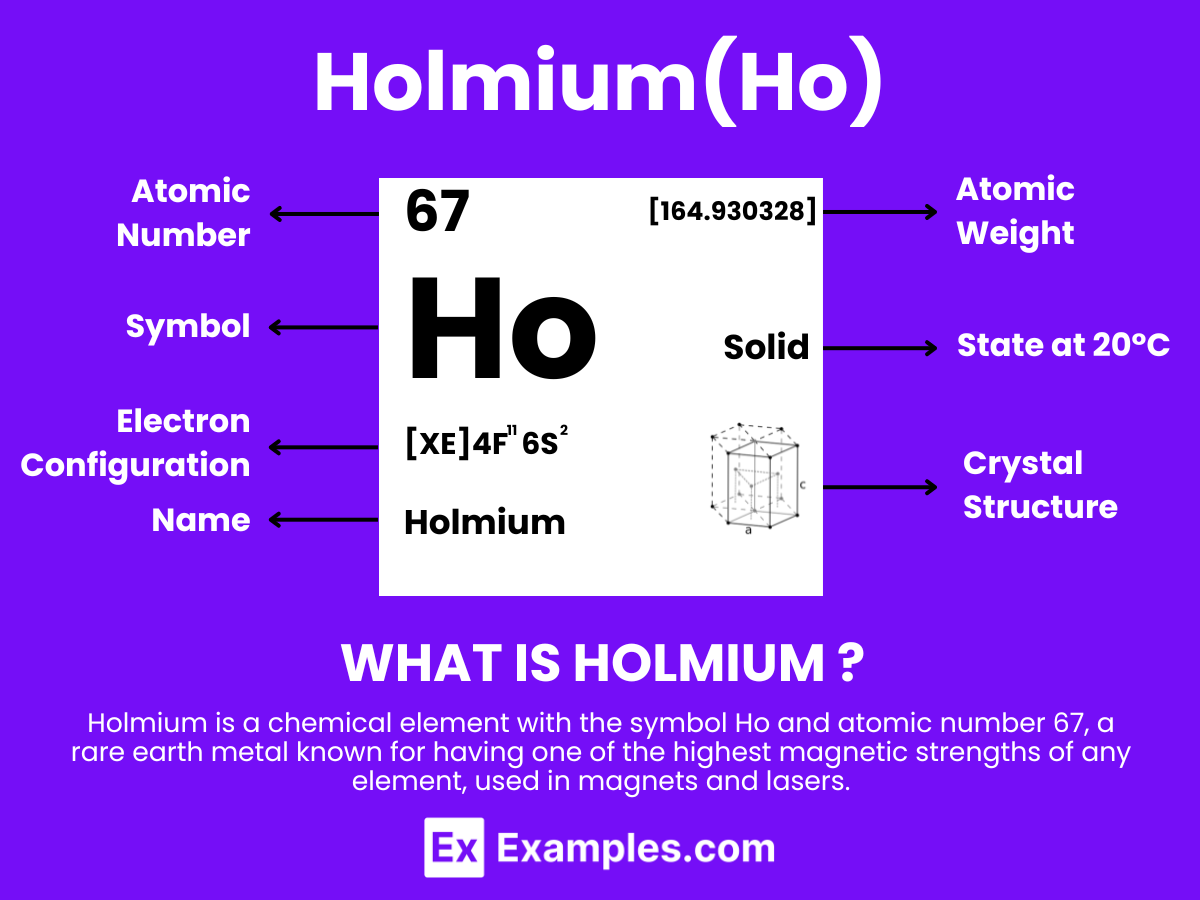
Holmium is a chemical element with the symbol Ho and atomic number 67. It is part of the lanthanide series in the periodic table, which is comprised of rare earth elements. Holmium was discovered by Swiss chemist Marc Delafontaine and Swedish chemist Per Teodor Cleve in 1878. The element was named after Stockholm, Sweden (Holmia in Latin), the hometown of Cleve.
Characterized by its metallic, bright silvery luster, holmium is relatively soft and malleable. It has some of the highest magnetic properties of any element and significantly enhances the magnetic strength of magnets when included in their alloys. Despite being classified as a rare earth element, holmium is not exceptionally rare in the Earth’s crust, but it is difficult to separate from other lanthanides with which it naturally occurs.
Holmium, in contrast to hydrogen, is a metallic element with physical and chemical characteristics that reflect its position as a rare earth element in the lanthanide series of the periodic table. Unlike hydrogen, which is a gas under standard conditions and forms simple diatomic molecules (H₂), holmium displays the typical properties of metals, including malleability, ductility, and conductivity. Its behavior at the atomic and molecular levels significantly differs from that of hydrogen, given its larger atomic size, different electron configuration, and its metallic characteristics.
Atomic Level: Each holmium atom (Ho) contains 67 protons in its nucleus and is expected to have 67 electrons orbiting around it. The electron configuration of holmium is [Xe] 4f¹¹ 6s², indicating it has a relatively complex electron configuration with potential for various oxidation states, typical of lanthanide elements. This suggests a certain level of chemical reactivity, enabling it to form compounds with a variety of elements. Holmium’s properties are largely determined by its partially filled 4f electron shell, which contributes to its unique magnetic properties among other characteristics.
Molecular Formation: Unlike hydrogen, holmium does not form simple molecules through covalent bonding due to its metallic nature. In its solid form, holmium atoms are part of a metallic lattice structure characteristic of metals. This structure involves metallic bonding, where electrons are delocalized over many holmium atoms, differing fundamentally from the discrete electron sharing seen in hydrogen’s covalent bonds. Holmium’s interaction with other elements to form compounds typically involves ionic or metallic bonding, depending on the nature of the other element involved. Given its metallic state, holmium is used in various alloys and has applications in the production of magnets, nuclear reactors, and certain types of lasers, leveraging its unique properties such as high magnetic susceptibility.
| Property | Value |
|---|---|
| Atomic Number | 67 |
| Atomic Weight | 164.93033 u |
| Melting Point | 1474 °C (2685 °F) |
| Boiling Point | 2700 °C (4892 °F) |
| Density | 8.79 g/cm³ at 20 °C |
| State at 20 °C | Solid |
| Electronic Configuration | [Xe] 4f^11 6s^2 |
| Oxidation States | +3 (most common), +2 |
| Crystal Structure | Hexagonal Close-Packed (hcp) |
| Thermal Conductivity | 16 W/(m·K) at 25 °C |
| Electrical Resistivity | 814 nΩ·m at 20 °C |
| Magnetic Ordering | Paramagnetic |
Holmium, like other lanthanides, primarily exhibits a +3 oxidation state in its compounds. It is fairly reactive and will tarnish slowly in air, forming the oxide Ho2O3. When heated, holmium reacts with water vapor to form hydroxides. It reacts with all halogens to form trihalides:
Holmium dissolves readily in dilute sulfuric acid to form solutions containing the yellow Ho(III) ions, which exist as [Ho(OH₂)₉]³⁺ complexes
| Property | Value |
|---|---|
| Standard Atomic Weight | 164.93033 g/mol |
| Enthalpy of Atomization | 317 kJ/mol (at 25 °C) |
| Enthalpy of Fusion | 17 kJ/mol |
| Enthalpy of Vaporization | 265 kJ/mol |
| Standard Molar Entropy (S°298) | 64.8 J/(mol·K) |
| Heat Capacity (Cp) | 27.15 J/(mol·K) at 25 °C |
| Property | Value |
|---|---|
| Young’s Modulus | 64.8 GPa |
| Shear Modulus | 26.3 GPa |
| Bulk Modulus | 40.2 GPa |
| Poisson’s Ratio | 0.231 |
| Mohs Hardness | ~4.5 |
| Vickers Hardness | 410 MPa |
| Brinell Hardness | 600 MPa |
| Thermal Expansion | 11.2 µm/(m·K) at 25 °C |
| Physical Properties | Chemical Properties |
|---|---|
| Atomic Number: 67 | Oxidation States: +3 |
| Atomic Weight: 164.93033 | Electronegativity: 1.23 (Pauling scale) |
| Density: 8.79 g/cm³ at 20°C | Common Ions: Ho³⁺ |
| Melting Point: 1474°C | Reactivity: Moderately reactive, especially when powdered |
| Boiling Point: 2700°C | Solubility: Insoluble in water, soluble in acids |
| Electrical Resistivity: 81.0 microohms·cm at 0°C | Affinity for Oxygen: Forms oxides readily when exposed to air |
| Physical Properties | Chemical Properties |
|---|---|
| Natural Isotopes: Ho-165 | Neutron Cross Section: 64 barns for thermal neutrons |
| Half-Life: Stable (Ho-165) | Neutron Mass Absorption: 0.023 |
| Radioactive Isotopes: Several, including Ho-163 and Ho-166 with short half-lives | Isotopic Abundance: Ho-165 is 100% |
| Magnetic Dipole Moment: 10.6 µB (for Ho-165) | |
| Specific Heat Capacity: 27.15 J/mol·K | |
| Thermal Conductivity: 16.2 W/(m·K) |
The preparation of holmium from its ores involves a multi-step process due to its occurrence with other lanthanides from which it must be separated. The primary source of holmium is monazite and bastnäsite, two rare earth minerals that contain a variety of lanthanides including holmium. Here’s a simplified overview of how holmium is typically prepared, with examples of processes and techniques used at various stages:
| Isotope | Half-life | Decay Mode |
|---|---|---|
| Ho-140 | Unknown | Alpha decay |
| Ho-141 | Unknown | Alpha decay |
| Ho-142 | Unknown | Alpha decay |
| Ho-143 | Unknown | Alpha decay |
| Ho-144 | Unknown | Alpha decay |
| Ho-145 | Unknown | Alpha decay |
| Ho-146 | 3.6 seconds | Beta decay |
| Ho-147 | 5.8 seconds | Beta decay |
| Ho-148 | >1 second | Beta decay |
| Ho-149 | 21 seconds | Beta decay |
| Ho-150 | 76.8 seconds | Beta decay |
| Ho-151 | 35 seconds | Beta decay |
| Ho-152 | >1 minute | Beta decay |
| Ho-153 | 2 minutes | Beta decay |
| Ho-154 | 11.76 minutes | Beta decay |
| Ho-155 | 48 minutes | Beta decay |
| Ho-156 | 56 minutes | Beta decay |
| Ho-157 | 12.6 minutes | Beta decay |
| Ho-158 | 11 minutes | Beta decay |
| Ho-159 | 33.05 minutes | Beta decay |
| Ho-160 | 25.6 minutes | Beta decay |
| Ho-161 | 2.48 hours | Beta decay |
| Ho-162 | 15 minutes | Beta decay |
| Ho-163 | Stable | – |
| Ho-164 | 29 minutes | Beta decay |
| Ho-165 | Stable | – |
| Ho-166m | 1,200 years | Isomeric transition, β- |
| Ho-166 | 26.8 hours | Beta decay |
| Ho-167 | 3 hours | Beta decay |
| Ho-168 | 3 minutes | Beta decay |
| Ho-169 | 4.72 minutes | Beta decay |
| Ho-170 | Unknown | Beta decay |
| Ho-171 | Unknown | Beta decay |
| Ho-172 | Unknown | Beta decay |
| Ho-173 | Unknown | Beta decay |
| Ho-174 | Unknown | Beta decay |
| Ho-175 | Unknown | Beta decay |
The production of holmium involves a series of complex processes, starting from the extraction of rare earth minerals to the separation, reduction, and refinement of holmium into a usable form. The process typically follows these steps:
Holmium’s unique properties make it useful in a variety of applications, ranging from nuclear to medical technologies:
Article provides a comprehensive overview of holmium, from its extraction and production processes to its diverse applications. Highlighting holmium’s role in advanced technologies and its unique properties, such as strong magnetic fields and precision in medical lasers, the piece underscores the element’s significance in scientific research and various industrial sectors, showcasing the continuous need for its efficient utilization.
Text prompt
Add Tone
Production of Holmium
Applications of Holmium
What is the atomic number of Holmium?
64
65
66
67
What is the chemical symbol for Holmium?
Hm
Ho
Hn
Hl
Holmium belongs to which group of elements?
Alkali metals
Alkaline earth metals
Lanthanides
Actinides
Holmium is primarily used in which application?
Fertilizers
Magnets
Jewelry
Fuel additives
What is the color of Holmium in its pure form?
Silver
Gold
Gray
White
Which property of Holmium makes it useful in nuclear reactors?
High thermal conductivity
High neutron absorption cross-section
High melting point
Low density
Holmium is known for having the highest magnetic moment of any naturally occurring element. What is this value?
7.5 μB
10.6 μB
13.3 μB
14.7 μB
What is the melting point of Holmium?
950°C
1074°C
1470°C
1650°C
Holmium can form which type of compounds?
Halides
Oxides
Sulfides
All of the above
What is the crystal structure of Holmium at room temperature?
Face-centered cubic
Body-centered cubic
Hexagonal close-packed
Simple cubic
Before you leave, take our quick quiz to enhance your learning!

