What is the atomic number of Indium?
48
49
50
51
Indium, a lesser-known yet intriguing element, offers a wealth of educational opportunities. With its unique properties and applications, this guide is tailored to help educators bring the wonders of Indium into the classroom. From its role in technology to its place on the periodic table, we provide engaging examples and insights. Enhance your science curriculum with our Indium guide, designed to captivate and educate students about this fascinating metal.
What is Indium?
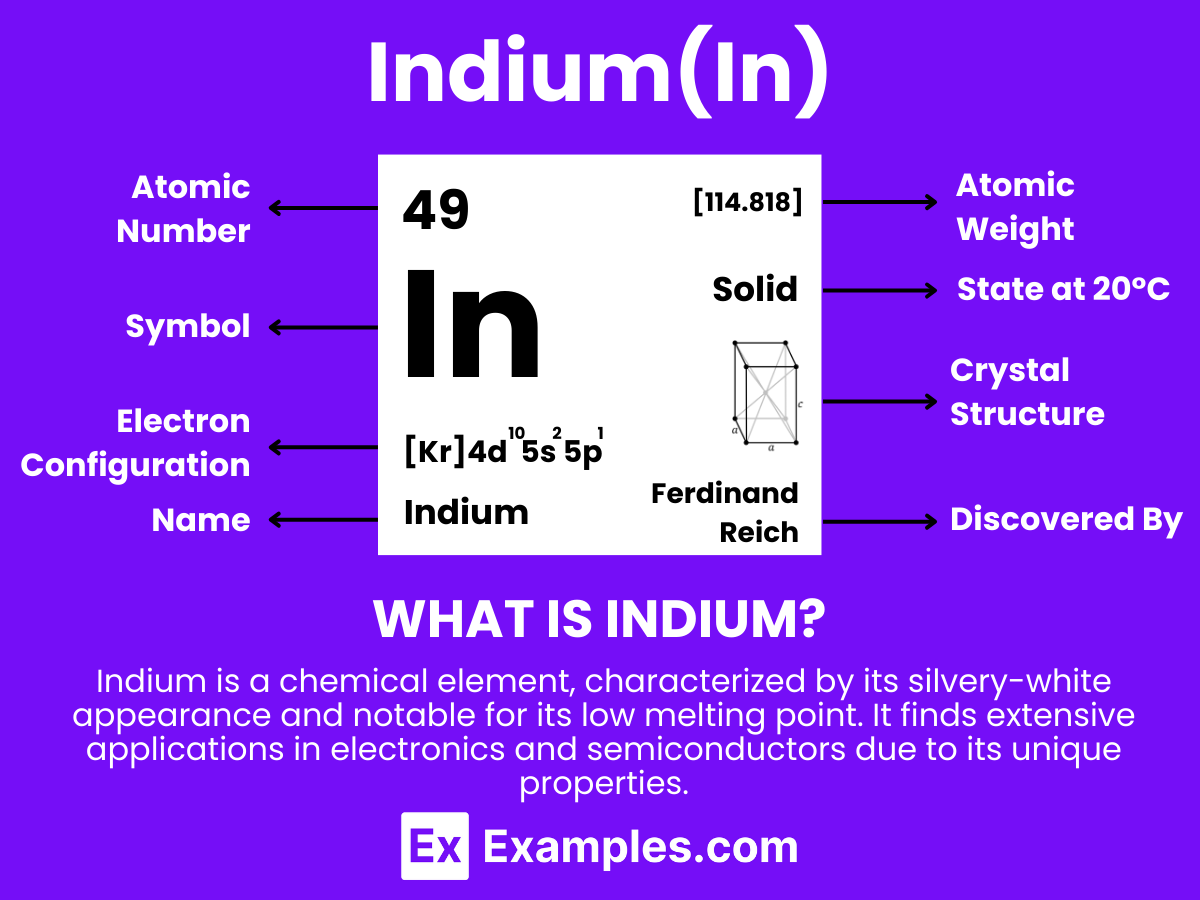
Indium is a chemical element with the symbol In and atomic number 49. It is a soft, malleable, and silvery-white metal that is commonly found in small amounts in zinc ores. Notably used in the production of LCD screens and solders, Indium has significant technological importance. Its low melting point and ability to adhere to glass make it valuable in various industrial applications. This element serves as an excellent teaching tool to illustrate concepts in chemistry and physics, particularly in discussions about metal properties and their roles in modern technology.
Indium metal (In) consists of atoms bonded together. Each indium atom has 49 protons in its nucleus and varying numbers of neutrons, depending on the isotope. In the indium solid, the atoms are closely packed in a metallic crystal lattice structure.
Atomic Level: Each indium atom (In) consists of 49 protons and a varying number of neutrons. Metallic Bonding: The indium atoms form metallic bonds with neighboring atoms, where outer electrons are delocalized and free to move throughout the metal lattice.
The bonding between the indium atoms is relatively strong due to metallic bonding, resulting in a solid metal with characteristic properties such as malleability, ductility, and conductivity. At room temperature, indium is a silvery-white, malleable metal with a relatively low melting point, making it useful in various applications such as electronics, soldering, and coatings.
| Property | Detail |
|---|---|
| Appearance | Silvery-white, lustrous metal |
| State at Room Temperature | Solid |
| Density | 7.31 g/cm³, relatively low density |
| Melting Point | 156.60 °C, one of the lowest for metals |
| Boiling Point | 2,072 °C |
| Electrical Conductivity | Highly conductive, used in electronics |
| Thermal Conductivity | Moderately high, efficient heat transfer |
| Malleability and Ductility | Highly malleable and ductile, easily formed |
| Crystal Structure | Tetragonal, typical of post-transition metals |
| Hardness | Relatively soft, can be cut with a knife |
| Sound Speed | Speed of sound in indium is about 1215 m/s |
| Reflectivity | Highly reflective, especially in thin films |
Indium is a relatively rare, post-transition metal known for its softness and malleability. Chemically, it shares properties with both its group members, gallium and thallium.
| Isotope | Natural Abundance | Half-Life | Decay Mode |
|---|---|---|---|
| In-113 | 4.3% | Stable | – |
| In-115 | 95.7% | 4.41 x 10¹⁴ years | Beta decay to tin-115 (Sn-115) |
Indium has two naturally occurring isotopes. Indium-115, the more abundant isotope, is mildly radioactive but with a very long half-life, making it practically stable for most practical purposes. The stability and abundance of these isotopes make indium a reliable element in various industrial and scientific applications.
Indium, a lustrous, silvery metal, has several vital applications in various industries due to its unique properties like malleability, ductility, and ability to form alloys. Here are the top five uses of Indium:
Indium is a key component in indium tin oxide (ITO), which is used in touch screens and liquid crystal displays (LCDs). ITO is a transparent conductor, making it ideal for controlling screen pixels in electronic devices like smartphones, tablets, and televisions.
Indium is used in solders and alloys due to its low melting point and ability not to corrode over time. It is particularly useful in lead-free solders and alloys with other metals to improve their thermal fatigue performance, making it essential in electronics manufacturing.
Indium phosphide (InP) and indium arsenide (InAs) are used in semiconductors for high-speed and high-frequency electronics. These compounds are vital in the production of diodes, transistors, and integrated circuits.
Due to its excellent thermal conductivity, Indium is used as a thermal interface material in heat sinks and heat exchangers. It helps in efficient heat dissipation in electronic devices, preventing overheating.
Indium is used in the production of thin-film solar cells. Indium gallium arsenide (InGaAs) and copper indium gallium selenide (CIGS) are used in photovoltaic cells for converting solar energy into electricity, contributing to sustainable energy solutions.
The commercial production of Indium primarily involves extracting it as a by-product from the processing of other metals, notably zinc. Indium is not usually found in its pure form but as a trace element in various minerals. The production process typically includes the following steps:
Indium, while valuable in various industrial applications, poses certain health risks when exposure occurs, particularly in occupational settings. Understanding these health effects is crucial for ensuring safety and implementing appropriate protective measures.
Indium’s environmental impact, particularly due to mining and industrial use, is an area of growing concern, emphasizing the need for sustainable practices and effective waste management.
Indium is generally considered non-toxic to humans in small quantities. However, prolonged exposure or ingestion of large amounts can lead to health concerns, including gastrointestinal irritation and potential organ damage. Proper handling and disposal practices are recommended to minimize any potential risks associated with indium exposure
Indium finds versatile applications due to its unique properties. It is commonly used in the production of electronic components like semiconductors and liquid crystal displays (LCDs). Additionally, it serves as a crucial component in alloys, solders, and thin-film coatings, contributing to advancements in technology and various industrial processes
Yes, you can touch indium with your bare hands. Indium is a solid metal at room temperature and is generally safe to handle. However, it’s always advisable to wash your hands afterward to remove any potential contaminants, as with handling any other metallic substances.
In summary, indium is a versatile metal with unique properties that make it valuable in various industries. Its malleability, conductivity, and low melting point contribute to its widespread use in electronics, soldering, and coatings. Understanding the atomic and molecular structure of indium provides insight into its behavior and applications, highlighting its importance in modern technology and manufacturing processes.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Indium?
48
49
50
51
What is the chemical symbol for Indium?
In
Id
Im
Ir
Indium belongs to which group in the periodic table?
Group 12
Group 13
Group 14
Group 15
What is the most common oxidation state of Indium?
+1
+2
+3
+4
Indium is often used in which type of display technology?
LED
LCD
CRT
Plasma
What is the primary mineral source of Indium?
Sphalerite
Bauxite
Hematite
Galena
What is the melting point of Indium?
156.6°C
231.9°C
327.5°C
660.3°C
Indium forms alloys with which metal to create low-melting-point alloys?
Aluminum
Bismuth
Copper
Nickel
Which property of Indium makes it useful for creating seals in cryogenics?
High melting point
High density
Ductility
Low reactivity
In which year was Indium discovered?
1800
1863
1901
1937
Before you leave, take our quick quiz to enhance your learning!

