What is the atomic number of Livermorium?
114
115
116
117
Livermorium, a synthetic element with the symbol Lv, stands as a pinnacle of human ingenuity in the periodic table. This comprehensive guide delves into the depths of Livermorium, offering insightful examples that illuminate its definition, unique properties, practical applications, and intriguing compounds. By exploring the nuances of Livermorium, readers gain a thorough understanding of this enigmatic element, enhancing their knowledge of modern chemistry and its innovative advancements. Join us as we uncover the fascinating world of Livermorium, showcasing its significance in scientific research and potential uses.
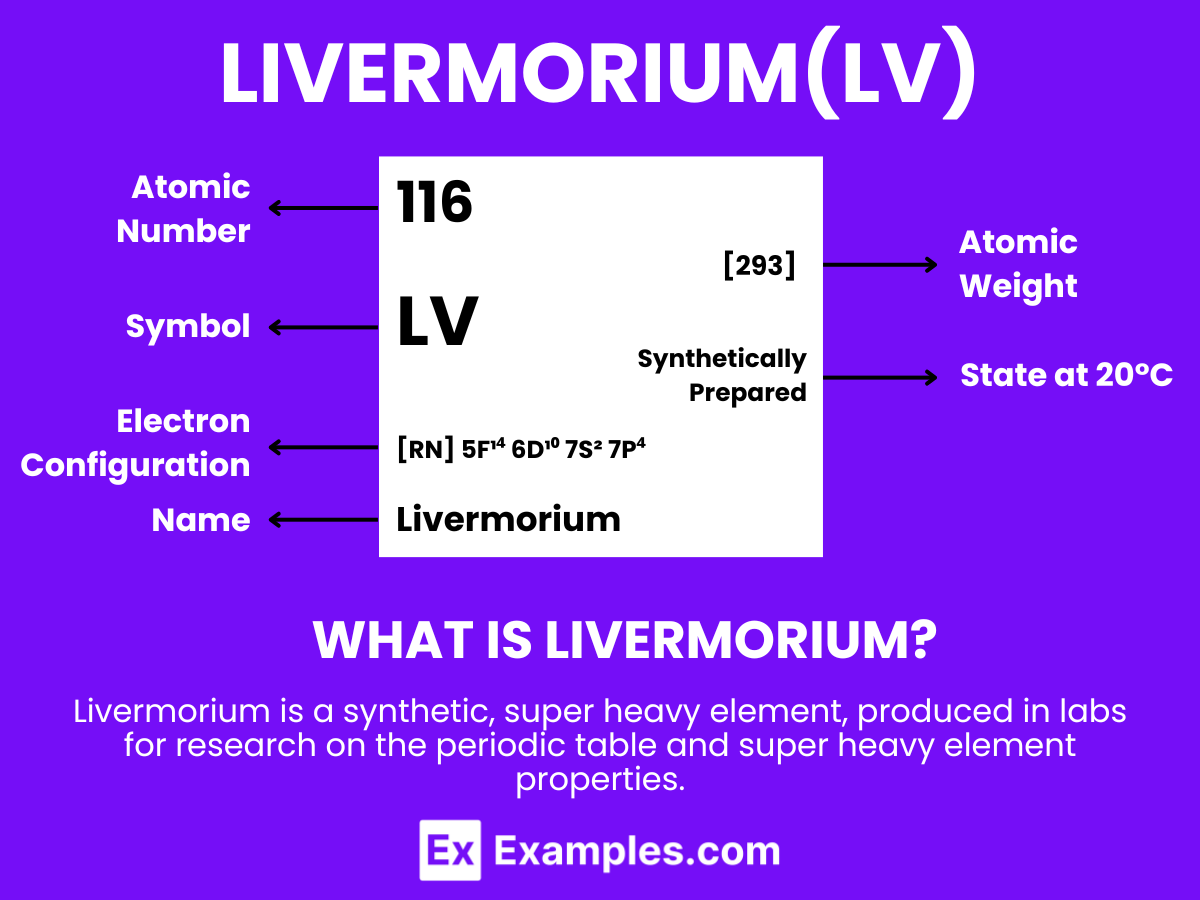
Livermorium is a superheavy, synthetic element with the chemical symbol Lv and atomic number 116. It is notable for being created in particle accelerators by fusing atomic nuclei. Livermorium does not occur naturally and exists only briefly before decaying, making it challenging to study. Its discovery is significant for research in nuclear physics, particularly in understanding the properties and behaviors of superheavy elements in the periodic table. Due to its extreme instability and radioactivity, livermorium has no practical applications outside scientific research, where it contributes to exploring the theoretical “island of stability” and the limits of the periodic table.
Livermorium, a synthetic element far removed from the lighter and more commonly encountered elements like hydrogen or gallium, is a super heavy element that occupies a unique position in nuclear chemistry due to its place in the periodic table and its classification as a post-actinide.
Atomic Level: Each atom of Livermorium (Lv) is characterized by having 116 protons in its nucleus, a feature that defines its atomic number as 116. The theoretical electron configuration of Livermorium is [Rn]5f¹⁴ 6d¹⁰ 7s² 7p⁴ suggesting it has a full 5f and 6d orbital, with four electrons in its 7p orbital, poised for chemical interactions. However, relativistic effects are expected to influence its actual electron configuration, potentially altering its chemical properties.
Molecular Formation: Unlike simpler elements that can form diatomic molecules (such as H₂), Livermorium does not naturally form molecules or exhibit a stable molecular structure due to its extremely short half-life and high instability. The element exists for mere milliseconds before decaying into lighter elements, making the study of its bonding characteristics and molecular formation largely theoretical. In the hypothetical scenario where Livermorium atoms could persist long enough to interact chemically, their behavior would likely be influenced by their electron configuration, but this remains speculative.
The stability and phase of Livermorium under various temperatures and pressures are subjects of theoretical speculation, as its brief existence precludes the observation of solid, liquid, or gaseous states under normal conditions. The term “Livermorium Gas” does not apply in the same way it might for compounds like uranium hexafluoride (UF₆) in uranium’s context; Livermorium’s extreme radioactivity and rapid decay mean it has not been observed in compound form or any state other than as fleeting, individual atoms produced in particle accelerators.
| Property | Description |
|---|---|
| Appearance | Not observed directly; assumed to have no stable or long-lasting physical form due to its radioactivity |
| Atomic Number | 116 |
| Density (at 20°C) | 12.9 g/cm³ |
| Melting Point | 340-540°C |
| Boiling Point | 100°C |
| State at Room Temperature | Expected to be solid |
| Electron Configuration | [Rn] 5f¹⁴ 6d¹⁰ ₇s² ₇p⁴ |
| Common Oxidation States | +2, +4 |
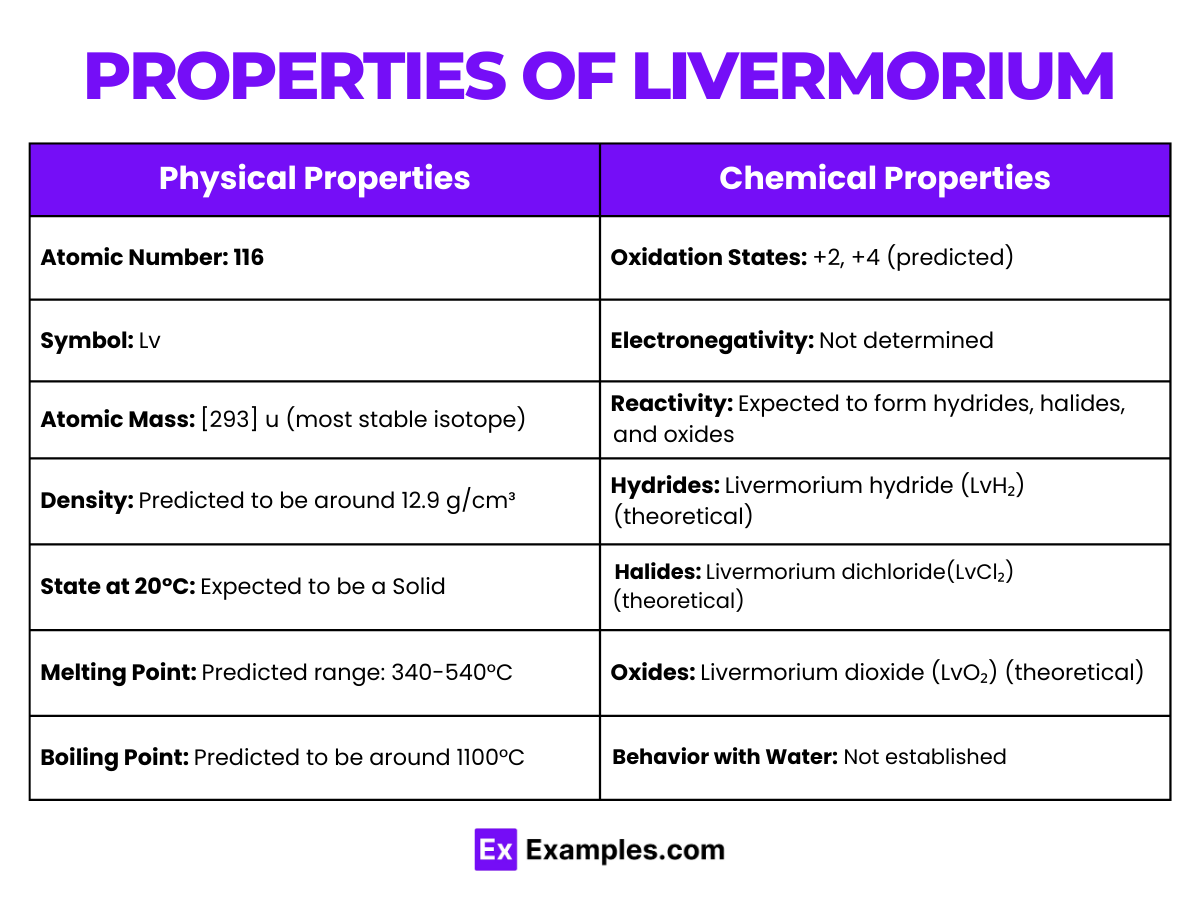
Livermorium, with the atomic number 116, is a synthetic element located in group 16 of the periodic table. Its chemical properties are primarily theoretical due to its extremely short half-life and the challenges associated with its production. Here, we explore the anticipated chemical properties of livermorium, based on its position in the periodic table and theoretical predictions.
The exploration of livermorium’s chemical properties remains largely theoretical, awaiting advancements in experimental techniques and the production of more stable isotopes for in-depth study.
| Property | Description |
|---|---|
| Standard State | Solid (expected) |
| Melting Point | 340-540°C (estimated range based on theoretical calculations) |
| Boiling Point | Approximately 1100°C (predicted) |
| Property | Value with Unit |
|---|---|
| Density (at 20°C) | Estimated to be around 12.9 g/cm³(theoretical) |
| Viscosity | Not Applicable (Solid, theoretical) |
| Solubility in Water | Insoluble (theoretical) |
| Color | Not observed directly; no stable or visible appearance due to rapid decay |
| Phase at Room Temperature | Expected to be solid (theoretical) |
| Property | Value with Unit |
|---|---|
| Atomic Number | 116 |
| Atomic Mass | Most stable isotope: Livermorium-293 (293 u) |
| Isotopes | ^293Lv (most stable), ^292Lv, ^291Lv, among others |
| Half-Life (for ^293Lv) | ~60 milliseconds |
| Half-Life (for ^292Lv) | ~20 milliseconds |
| Half-Life (for ^291Lv) | ~18 milliseconds |
| Nuclear Spin | Not precisely determined due to short half-lives |
| Neutron Cross Section | Not determined (extremely short-lived isotopes make measurement challenging) |
Livermorium is a super heavy, synthetic element that is not found naturally and can only be created in a laboratory setting. The preparation of livermorium involves highly specialized equipment, advanced nuclear reactors, and ion accelerators. Below is an outline of the general process used to create livermorium:
Livermorium is a synthetic element with no stable isotopes. It has several isotopes that have been identified through nuclear reactions, each exhibiting unique decay properties.
| Isotope | Half-Life | Decay Mode |
|---|---|---|
| Lv-290 | 8 milliseconds | Alpha decay to Fl-286 |
| Lv-291 | ~18 milliseconds | Alpha decay to Fl-287 |
| Lv-292 | ~20 milliseconds | Alpha decay to Fl-288 |
| Lv-293 | ~60 milliseconds | Alpha decay to Fl-289 |
| Lv-294 | ~40 milliseconds | Alpha decay to Fl-290 |
| Lv-295 | Predicted, not observed | Predicted alpha decay |
| Lv-296 | Predicted, not observed | Predicted alpha decay |
Livermorium is a synthetic, superheavy element with the atomic number 116. Due to its extremely short half-life and the fact that it can only be produced in minute quantities. Below are the potential uses and areas of interest related to livermorium:
Livermorium, with the symbol Lv and atomic number 116, is a synthetic element produced in extremely small amounts through nuclear reactions involving heavy ions.
Livermorium is a synthetic element with the atomic number 116, identified by its symbol, Lv.
livermorium is a synthetic, super heavy element marked by its elusive nature and scarcity of data. Primarily used for scientific research, its synthesis offers insights into the periodic table’s limits and nuclear physics. Despite lacking practical applications, livermorium’s study propels advancements in understanding super heavy elements and contributes to the quest for the island of stability.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Livermorium?
114
115
116
117
In which group of the periodic table is Livermorium located?
Group 16
Group 15
Group 14
Group 17
Which element is directly above Livermorium in the periodic table?
Polonium
Tellurium
Bismuth
Radon
What is the most stable isotope of Livermorium?
Lv-290
Lv-292
Lv-293
Lv-294
Livermorium was first synthesized in which year?
2000
2002
2004
2006
Livermorium is named after which laboratory?
Lawrence Berkeley National Laboratory
Los Alamos National Laboratory
Lawrence Livermore National Laboratory
Oak Ridge National Laboratory
What type of element is Livermorium?
Metal
Metalloid
Non-metal
Noble gas
What is the symbol for Livermorium?
Lv
Lm
Lr
Ll
Livermorium belongs to which period of the periodic table?
Period 6
Period 7
Period 8
Period 9
Livermorium was discovered by scientists from which two countries?
United States and Germany
United States and Russia
United States and Japan
United States and France
Before you leave, take our quick quiz to enhance your learning!

