What is the atomic number of Lutetium?
70
71
72
73
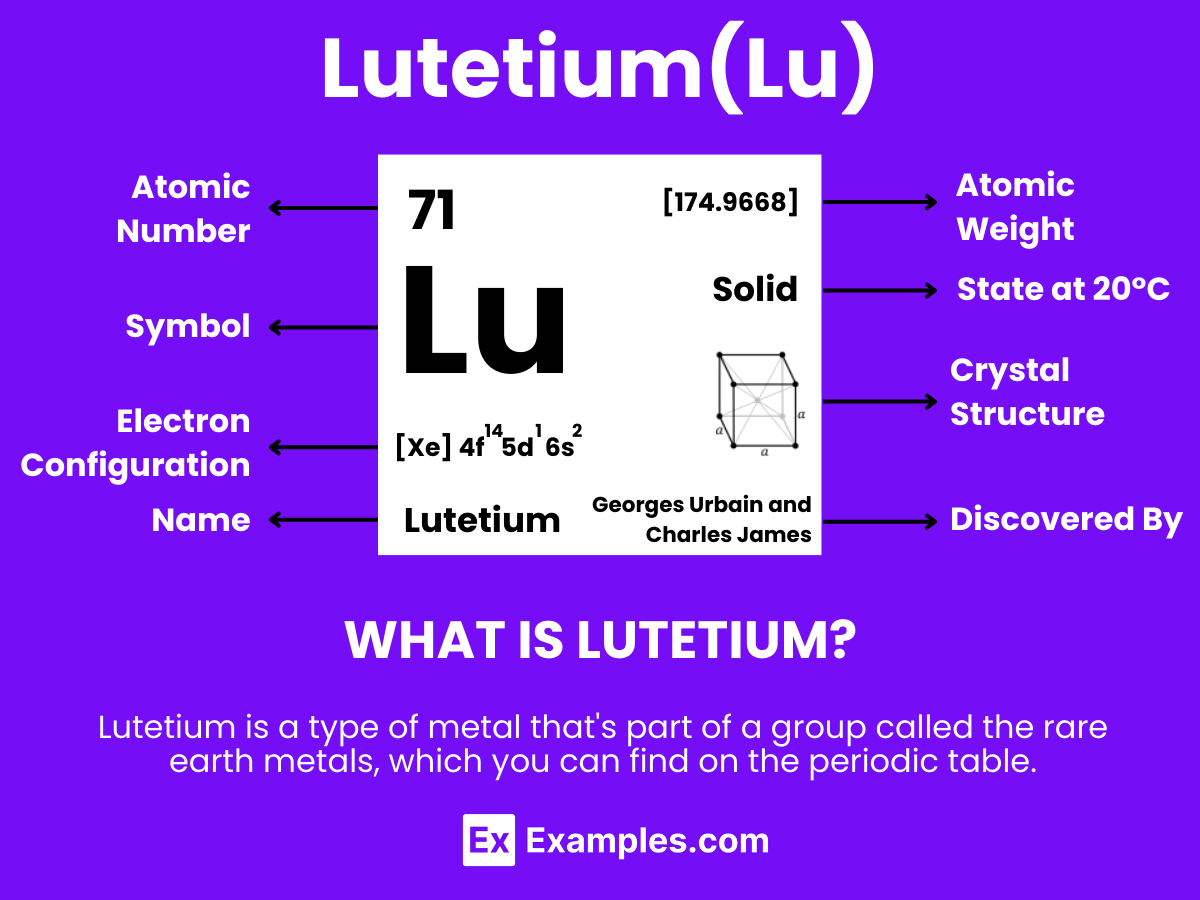
Lutetium, the last member of the lanthanide series, is a rare and intriguing element known for its unique chemical and physical properties. With its symbol Lu and atomic number 71, it stands out in the periodic table as a metal that is not only rare in nature but also invaluable in various high-tech and medical applications. This silvery-white element, discovered in the early 20th century, has since carved a niche for itself in the fields of science and technology, offering remarkable contributions to our understanding of materials and their capabilities
Lutetium is a type of metal that’s part of a group called the rare earth metals, which you can find on the periodic table. It’s known by the symbol “Lu” and has the atomic number 71. Even though it’s called “rare,” it’s actually more common than some of the other elements in its group. Lutetium looks silvery-white and is known for being the heaviest, hardest, and having the highest melting point among its rare earth siblings.
| Property | Value |
|---|---|
| Appearance | Silvery white, hard metal |
| Atomic Number | 71 |
| Atomic Weight | 174.9668 u |
| Density | 9.841 g/cm³ |
| Melting Point | 1663 °C (3025 °F) |
| Boiling Point | 3402 °C (6156 °F) |
| State at 20 °C | Solid |
| Electrical Conductivity | Good, typical of metals |
| Thermal Conductivity | 16.4 W/(m·K) |
| Oxidation States | +3, the most stable state |
| Electronegativity | 1.27 (Pauling scale) |
| Heat of Fusion | 22 kJ/mol |
| Heat of Vaporization | 414 kJ/mol |
| Specific Heat Capacity | 154 J/(kg·K) |
| Crystal Structure | Hexagonal close-packed (hcp) |
Lutetium, with the symbol Lu and atomic number 71, has several chemical properties that make it stand out among the elements, especially within the lanthanide series. Here are some of its notable chemical properties:
The most common oxidation state of lutetium is +3. This characteristic is shared among many lanthanides, which tend to lose three electrons to form stable trivalent ions. This +3 oxidation state is pivotal in defining the chemical behavior and reactivity of lutetium in various compounds.
Lutetium exhibits relative stability in dry air but can tarnish when exposed to moist air. This change indicates its reactivity with water vapor to form lutetium oxide. When lutetium reacts with water, the chemical equation can be represented as:
2Lu+6H₂O→2Lu(OH)₃+3H₂
This equation shows that lutetium reacts slowly with water to form lutetium hydroxide (Lu(OH)_₃), and the reaction rate increases with temperature.
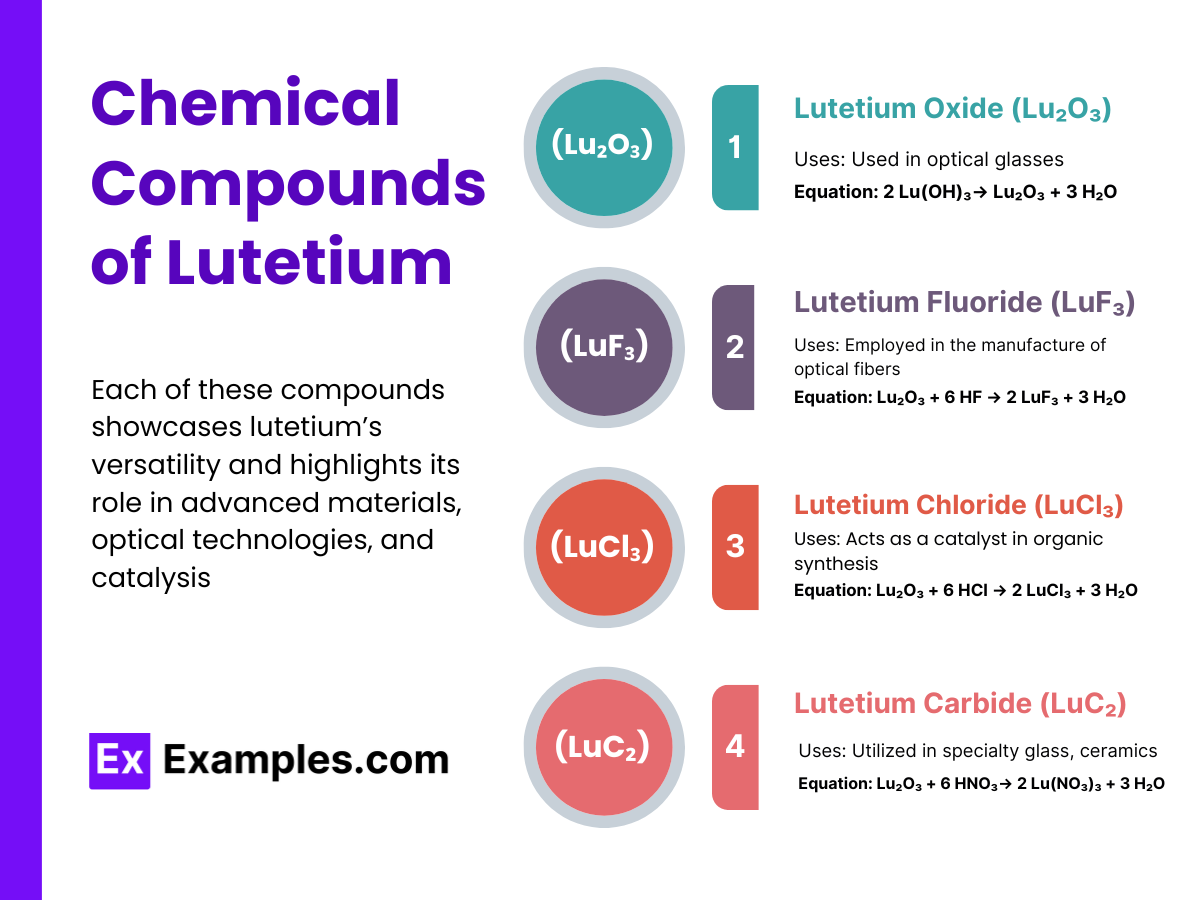
Lutetium forms a variety of compounds, predominantly in the +3 oxidation state. Significant examples include:
These compounds have considerable applications in various industrial and technological fields.
Lutetium possesses an electronegativity of 1.27 on the Pauling scale. This value indicates its relatively low ability to attract electrons towards itself within a chemical bond, typical for metals, especially those in the lanthanide series.
The electron configuration of lutetium is [Xe] 4f¹⁴5d¹6s². This distinctive configuration, featuring a completely filled 4f subshell, underscores its unique chemical properties and marks the end of the lanthanide series.
Generally, lutetium compounds are stable at room temperature. They can engage in chemical reactions under certain conditions, such as exposure to high temperatures or reactions with strong acids. These conditions underline lutetium’s chemical versatility and its ability to participate in various chemical processes.
| Property | Value |
|---|---|
| Standard Atomic Weight | 174.9668 u |
| Melting Point | 1663 °C (3025 °F) |
| Boiling Point | 3402 °C (6156 °F) |
| Heat of Fusion | 22 kJ/mol |
| Heat of Vaporization | 414 kJ/mol |
| Specific Heat Capacity | 154 J/(kg·K) |
| Thermal Conductivity | 16.4 W/(m·K) |
| Thermal Expansion | (at 25 °C) 9.9 µm/(m·K) |
| Entropy of Formation | (Standard molar entropy, S°) Not readily available |
| Gibbs Free Energy of Formation | (Standard Gibbs free energy of formation, ΔfG°) Not readily available |
| Property | Value |
|---|---|
| Crystal Structure | Hexagonal close-packed (hcp) |
| Melting Point | 1663 °C (3025 °F) |
| Boiling Point | 3402 °C (6156 °F) |
| Density | 9.841 g/cm³ at 20 °C |
| Atomic Mass | 174.967 u |
| Atomic Number | 71 |
| Thermal Conductivity | 16.4 W/(m·K) |
| Thermal Expansion | 9.9 µm/(m·K) (at 25 °C) |
| Electrical Resistivity | 582 nΩ·m (at 20 °C) |
| Young’s Modulus | 68.6 GPa |
| Shear Modulus | 27.2 GPa |
| Bulk Modulus | 47.6 GPa |
| Mohs Hardness | ~2.6 |
| Vickers Hardness | 491-589 MPa |
| Brinell Hardness | 893 MPa |
| Specific Heat Capacity | 154 J/(kg·K) |
| Electronegativity | 1.27 (Pauling scale) |
| Common Oxidation States | +3 |
| Property | Description |
|---|---|
| Magnetic Ordering | Paramagnetic at room temperature |
| Magnetic Susceptibility | Positive, indicating attraction to magnetic fields |
| Electrical Conductivity | Good conductor of electricity, typical for metals |
| Superconducting Properties | Becomes a superconductor at very low temperatures, below 0.1 K |
| Optical Properties | Reflective and lustrous, with specific optical applications in laser materials |
| Nuclear Magnetic Resonance | Has isotopes suitable for NMR studies, useful in research and magnetic resonance imaging |
| Property | Value |
|---|---|
| Natural Isotopes | Lutetium-175 (stable), Lutetium-176 (stable) |
| Radioisotopes | Lutetium-177, Lutetium-174, among others |
| Half-Life of Lutetium-177 | 6.65 days |
| Neutron Cross Section | Lutetium-175: 74 barns (thermal neutrons) |
| Neutron Capture | Lutetium-176 has a high neutron capture ability, making it useful in nuclear technology |
| Use in Medicine | Lutetium-177 is used in targeted cancer therapy due to its beta emissions |
| Radiation Type | Beta emitter (Lutetium-177) |
| Decay Mode | Beta decay for most radioisotopes |
| Isotope | Half-Life | Decay Mode | Applications |
|---|---|---|---|
| Lutetium-173 | 1.37 years | Beta decay | Research |
| Lutetium-174 | 3.31 years | Beta decay | Research, Nuclear Physics |
| Lutetium-175 | Stable | N/A | Natural abundance, no significant applications due to stability |
| Lutetium-176 | 3.78 x 10^10 years | Beta decay | Geological dating, nuclear reactors |
| Lutetium-177 | 6.65 days | Beta decay | Targeted radiotherapy in medicine |
| Lutetium-178 | 23.1 minutes | Beta decay | Research |
| Lutetium-179 | 4.67 hours | Beta decay | Research |
Lutetium, a rare earth element, boasts several unique applications across various fields due to its distinctive properties. Here are some of its key uses:
The production of lutetium, a rare earth metal with distinctive properties, involves a series of complex and carefully controlled processes. Lutetium is the heaviest and hardest of the rare earth elements, making its production not only challenging but also crucial for its various applications in technology, medicine, and industry. Here’s an extensive look into the production of lutetium, from the initial extraction to the final purification stages.
Lutetium is typically extracted from mineral ores such as monazite and xenotime, which contain a mixture of rare earth elements.
After initial processing, the ore undergoes chemical treatment to extract lutetium and other rare earth elements.
These techniques further purify the rare earth elements, including lutetium, by exploiting differences in their chemical behavior.
Once lutetium is purified as an ion in solution, it needs to be converted into its metallic form.
The produced lutetium metal may undergo further refining to remove any remaining impurities. It can also be alloyed with other metals to enhance its properties for specific applications.
Lutetium, the heaviest and hardest of the rare earth elements, occupies a unique niche in modern technology and medicine due to its distinct physical and chemical properties. With its atomic number 71, lutetium is not just a fascinating subject for academic research but also a valuable component in various applications across multiple industries. Here’s a comprehensive overview of lutetium’s applications, emphasizing its importance and utility.
Lutetium serves as an efficient catalyst in a wide range of chemical reactions. Its use in catalysis is marked by the following points:
One of the most notable applications of lutetium is in the medical field, particularly in cancer treatment.
Lutetium’s optical and electrical properties make it valuable in the electronics and optoelectronics industries.
Lutetium’s stable and radioactive isotopes have applications in nuclear physics and geological research.
Lutetium contributes to the development of new materials with superior properties.
The unique properties of lutetium fuel ongoing research and innovation in several cutting-edge technologies.
In Conclusion, Lutetium, a rare earth metal with unique properties, plays a crucial role in advanced technologies, including cancer treatment, electronics, and materials science. Its rarity and specialized applications highlight its significance in scientific and industrial fields.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Lutetium?
70
71
72
73
What is the chemical symbol for Lutetium?
L
Lu
Lt
Le
Lutetium belongs to which group of elements in the periodic table?
Alkali metals
Transition metals
Lanthanides
Actinides
What is the standard atomic weight of Lutetium?
175.94
172.94
177.96
174.97
Lutetium is commonly used in which field?
Agriculture
Medicine
Electronics
Construction
Which isotope of Lutetium is used in targeted cancer therapy?
Lu-175
Lu-176
Lu-177
Lu-178
Lutetium is typically extracted from which type of minerals?
Sulfide minerals
Halide minerals
Phosphate minerals
Carbonate minerals
What is the melting point of Lutetium?
942°C
1663°C
1770°C
1925°C
Which property makes Lutetium suitable for use in PET (Positron Emission Tomography) scans?
High density
High atomic number
Radioactive isotopes
Low melting point
Lutetium is the heaviest and hardest member of which series?
Actinide series
Alkali metals
Transition metals
Lanthanide series
Before you leave, take our quick quiz to enhance your learning!

