What is the atomic number of Manganese?
23
25
27
29
Manganese, a pivotal element in the periodic table, plays a crucial role in a myriad of industrial applications, ranging from steel production to battery technology. This comprehensive guide delves into the versatile uses, benefits, and the pivotal role manganese occupies in modern industries. With a focus on practical examples, we illuminate how this essential element not only strengthens alloys but also contributes to the green energy sector. Stay tuned as we explore the multifaceted world of manganese, showcasing its significance across various sectors.
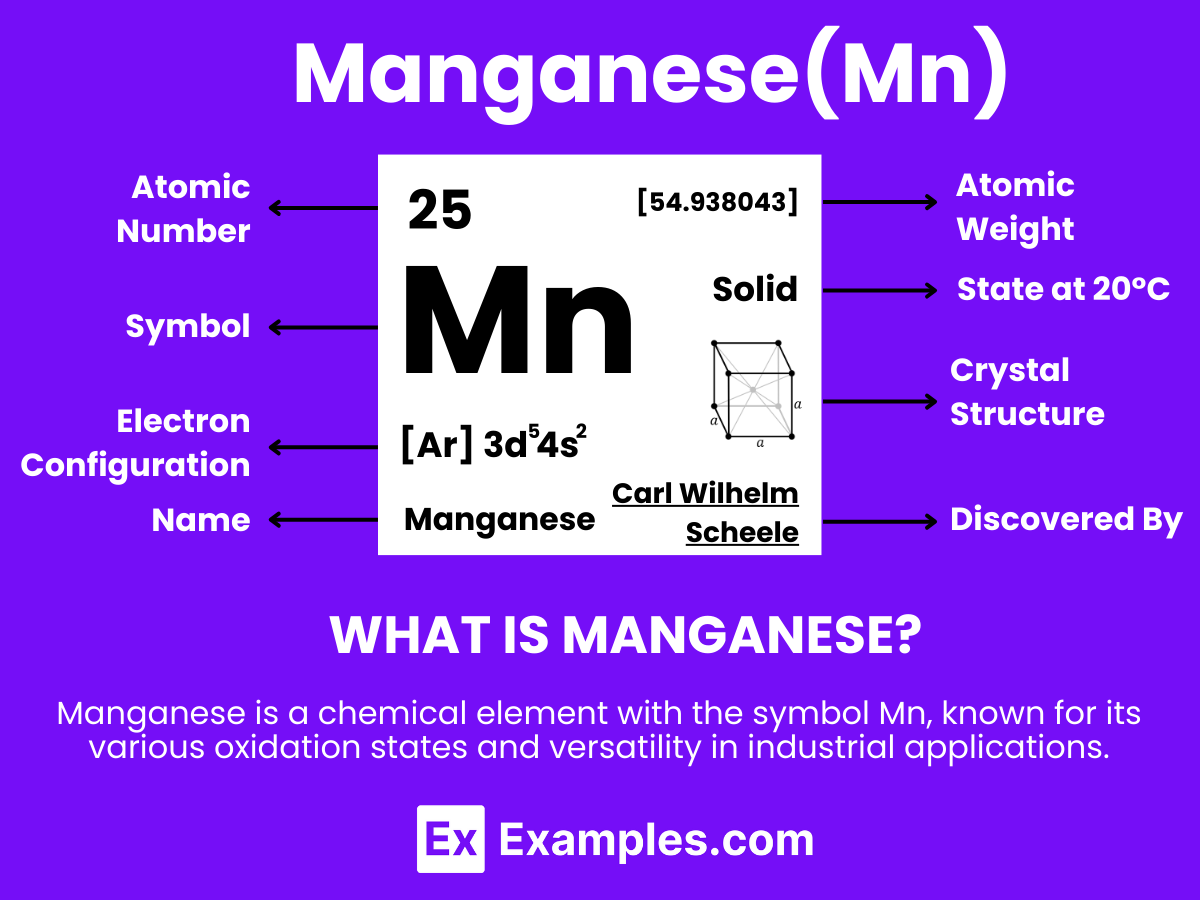
Manganese, a hard, silvery-gray metallic element with the atomic number 25, stands out for its remarkable properties and wide-ranging applications. Distinguished by its durability and resistance to wear, manganese is extensively used in the steel industry to improve the strength, hardness, and wear resistance of steel, making it indispensable in sectors like construction and automotive manufacturing. Additionally, manganese plays a crucial role in the chemical industry as a catalyst and is pivotal in the production of batteries through its use in manganese dioxide for alkaline batteries. This versatility and the critical role manganese plays across various industries highlight its significance in advancing technology and enhancing the materials used in our everyday lives.
As a pure element, manganese does not exhibit a molecular structure in the conventional sense, like molecules such as H₂O. At room temperature, manganese adopts a body-centered cubic (bcc) crystalline structure. This structural arrangement contributes to its characteristic properties and applications.
In compounds, manganese is known to share electrons covalently or to transfer electrons ionically, depending on the chemical nature of the elements it interacts with. This ability to participate in different types of bonding makes manganese a versatile element in chemistry.
Manganese is critically important due to its role in steel production, where it improves the strength and wear resistance of steel. It is also essential in the manufacture of batteries, particularly alkaline and lithium-ion batteries, due to its electrochemical properties. Moreover, manganese compounds are used as pigments and in water purification processes.
Manganese is pivotal in catalysis, playing a significant role in various chemical reactions and industrial processes. It is involved in the synthesis of organic compounds, functioning as a catalyst in environmental remediation and oxidation reactions. The diverse applications of manganese compounds in materials science, electronics, and sustainable technologies underscore its importance in modern chemical research and industry.
Manganese, with its intriguing atomic structure, stands as a cornerstone in the world of chemistry and materials science. This element, symbolized by Mn and possessing an atomic number of 25, showcases a unique arrangement of electrons that paves the way for its myriad of applications.
Protons, Neutrons, and Electrons: At the heart of manganese’s atomic structure lie 25 protons, nestled within the nucleus alongside a variable number of neutrons, typically around 30 for its most stable isotope. Surrounding the nucleus, 25 electrons whirl in orbit, organized into distinct energy levels or shells. This balanced distribution of protons and electrons gifts manganese with its characteristic chemical properties.
Electron Configuration: Manganese’s electron configuration, [Ar] 3d⁵ 4s², reveals a partially filled d-orbital, which is pivotal for its chemical behavior. This configuration results in manganese’s ability to form a wide variety of oxidation states, from -3 to +7, although +2, +4, and +7 are the most common. This flexibility in oxidation states underpins manganese’s role in redox reactions, catalysis, and its use in various compounds.
Atomic Radius and Ionic Properties: The atomic radius of manganese is approximately 161 picometers, a size that influences its bonding and interactions with other atoms and molecules. Manganese tends to form ionic compounds in its +2 oxidation state, such as manganese(II) chloride (MnCl₂), and covalent compounds in higher oxidation states, like manganese dioxide (MnO₂).
Significance in Materials and Biology: The atomic structure of manganese is not just a point of academic interest; it is directly linked to its utility in the world around us. Its diverse oxidation states allow for its use in steel production, batteries, and pigments. Beyond its industrial applications, manganese is vital in biological systems, acting as a cofactor for a variety of enzymes, playing a crucial role in photosynthesis, metabolism, and the detoxification of free radicals in organisms.
| Property | Value |
|---|---|
| Appearance | Silvery-gray metallic |
| Atomic Number | 25 |
| Atomic Mass | 54.938044 u |
| Density at 20°C | 7.3 g/cm³ |
| Melting Point | 1246°C (2275°F) |
| Boiling Point | 2061°C (3742°F) |
| State at 20°C | Solid |
| Electrical Conductivity | 6.2 × 10^6 S/m |
| Thermal Conductivity | 7.81 W/(m·K) |
| Heat of Fusion | 12.91 kJ/mol |
| Heat of Vaporization | 221 kJ/mol |
| Specific Heat Capacity | 26.32 J/(mol·K) |
Manganese showcases a variety of oxidation states, notably +2, +4, and +7. These states lead to diverse compounds:
Manganese reacts with oxygen, acids, and water, forming different manganese compounds:
Manganese is vital in redox reactions, especially in its +7 state as permanganate, which acts as a powerful oxidizing agent. In acidic solutions, it transforms to its +2 state, demonstrating its oxidizing ability.
Manganese is essential in photosynthesis and the earth’s redox cycle but can be harmful in excess, affecting the nervous system.
| Property | Value |
|---|---|
| Melting Point | 1519°C (2766°F) |
| Boiling Point | 2334°C (4233°F) |
| Heat of Fusion | 12.91 kJ/mol |
| Heat of Vaporization | 221 kJ/mol |
| Specific Heat Capacity | 26.32 J/(mol·K) |
| Thermal Conductivity | 7.81 W/(m·K) |
| Thermal Expansion | 21.7 µm/(m·K) (at 25°C) |
| Property | Value |
|---|---|
| Atomic Mass | 54.938045 u |
| Density | 7.44 g/cm³ (at 20°C) |
| Mohs Hardness | 6 |
| Young’s Modulus | 198 GPa |
| Bulk Modulus | 120 GPa |
| Poisson’s Ratio | 0.29 |
| Property | Value |
|---|---|
| Electrical Resistivity | 1.44 µΩ·m (at 20°C) |
| Magnetic Ordering | Paramagnetic |
| Curie Temperature | N/A (Paramagnetic at room temperature) |
| Magnetic Susceptibility | +850.0·10⁻⁶ cm³/mol |
| Property | Value |
|---|---|
| Isotopes | ^55Mn (Stable) |
| Atomic Number | 25 |
| Atomic Weight | 54.938045 |
| Half-life of Most Stable Isotope (^53Mn) | 3.7 million years |
| Neutron Cross Section | 13.3 barns (for ^55Mn) |
| Neutron Mass Absorption | 0.0018 |
The preparation of manganese involves several methods, primarily focusing on extracting and purifying the metal from its ores. Here’s a detailed overview of the main processes used in the preparation of manganese:
| Isotope | Mass Number | Natural Abundance (%) | Half-Life | Notes |
|---|---|---|---|---|
| Mn-52 | 52 | Synthetic | 5.591 days | Used in medical and research purposes |
| Mn-53 | 53 | Synthetic | 3.74 million years | Used for geological dating |
| Mn-54 | 54 | Synthetic | 312.2 days | Used in research |
| Mn-55 | 55 | 100 | Stable | Only stable isotope, common in nature |
| Mn-56 | 56 | Synthetic | 2.5789 hours | Used in medical research |
Manganese is a versatile metal with a wide range of industrial and chemical applications, vital for its physical and chemical properties.
In Steel Manufacturing: Manganese is crucial in steel production, improving hardness, durability, and resistance to wear. It acts as an alloying agent to enhance the strength and malleability of steel and iron.
Batteries: Manganese dioxide is extensively used in alkaline and zinc-carbon batteries as a cathode material, contributing to the battery’s stability and capacity.
Chemical Industry: Potassium permanganate, a manganese compound, is used as an oxidizing agent in chemical laboratories and water treatment processes due to its effectiveness in killing bacteria and removing odors.
Pigments: Manganese compounds, such as manganese dioxide, serve as pigments in ceramics and glass-making, imparting colors ranging from pink to green and violet.
Agriculture: Manganese is essential for plant growth, used as a micro-nutrient in fertilizers to correct manganese-deficient soils, thereby promoting healthy plant development.
The production of manganese involves several key processes, from mining to purification, aimed at producing high-quality manganese in various forms, suitable for industrial use.
Mining and Extraction: Manganese production begins with the extraction of manganese ore from mines. The most common manganese ores are pyrolusite (MnO₂) and rhodochrosite (MnCO₃). These ores undergo several stages of processing to increase the manganese content and remove impurities. The primary methods include crushing, screening, and washing, followed by dense media separation, jigging, and tabling.
Reduction Process: Once the ore is concentrated, it undergoes a reduction process, either through smelting in electric arc furnaces or through a more direct reduction. In the smelting process, the concentrated ore is mixed with coke and heated to high temperatures, where it reduces to manganese metal. The direct reduction process, often involving a rotary kiln, uses coal as a reductant to convert iron and manganese ores directly into metal.
Refining: The crude manganese produced through reduction is then refined to achieve higher purity levels. This refining process can include electrolytic methods, where manganese is dissolved in an electrolyte and deposited on a cathode, resulting in very pure manganese metal. Alternatively, thermal and chemical processes are used to produce different grades and forms of manganese, such as ferromanganese and silicomanganese, which are essential for steel production.
Manganese is a versatile metal with a wide range of applications, underscoring its importance across various industries.
Steel Production: The primary use of manganese is in the steel industry, where it serves as an alloying agent to improve the steel’s strength and resistance to wear and impact. About 90% of all manganese consumed annually goes into steel as ferromanganese and silicomanganese.
Batteries and Electronics: Manganese is crucial in the production of batteries, particularly alkaline and lithium-ion batteries, where it acts as a cathode material, providing essential properties for energy storage. Its role in electronics extends to various manganese compounds used in semiconductors, transistors, and other components.
Chemical Industry: In the chemical industry, manganese compounds are used in fertilizers, animal feed, and as a catalyst in the production of synthetic industrial chemicals. Manganese dioxide, for instance, is a key component in the manufacture of oxygen and chlorine and also serves as a depolarizer in dry cell batteries.
Environmental Applications: Manganese plays a role in environmental technology; for example, manganese oxides are used in water treatment facilities to remove impurities and in air purification systems to filter out toxic substances. Its ability to absorb pollutants makes it valuable in mitigating environmental contamination.
Manganese has intricately detailed the element’s physical and chemical properties, revealing its versatility and essential role in various industries. From steel production to advanced battery technology, manganese’s unique characteristics underscore its importance. Understanding these properties provides valuable insights into its applications and the innovative methods used in its preparation, highlighting manganese’s pivotal role in modern technological advancements.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Manganese?
23
25
27
29
What is the chemical symbol for Manganese?
Ma
Mn
Mg
Ms
Manganese is primarily obtained from which mineral?
Hematite
Bauxite
Pyrolusite
Galena
Which of the following is a common oxidation state of Manganese in its compounds?
+1
+2
+3
+4
What is the molar mass of Manganese?
54.94 g/mol
55.85 g/mol
56.94 g/mol
57.85 g/mol
Which alloy contains Manganese and is used to improve its hardness and strength?
Brass
Bronze
Steel
Duralumin
What is the primary use of Manganese in the steel industry?
As a reducing agent
To enhance corrosion resistance
To remove impurities
As an alloying element
What color is typically associated with Manganese compounds?
Red
Green
Purple
Blue
What is the melting point of Manganese?
1246°C
1519°C
1234°C
1341°C
Which of the following is NOT a characteristic of Manganese?
Hard
Brittle
Highly magnetic
Reactive
Before you leave, take our quick quiz to enhance your learning!

