What is the chemical symbol for Mercury?
Mg
Hg
H
Mn

Dive into the enigmatic world of mercury, a liquid metal that fascinates with its unique properties and wide-ranging applications. From ancient alchemical practices to modern scientific and industrial uses, mercury’s versatility is unmatched. This guide explores mercury’s role in thermometers, dental amalgams, and even in the extraction of gold and silver. Discover the intriguing aspects of mercury, including its compounds, safety measures, and environmental impact, through practical examples and insightful analysis. Embrace the liquid marvel that is mercury, understanding its essence and significance in our world.
Mercury is a unique, silvery liquid metal known for its remarkable properties and diverse range of applications, with the atomic number 80. It is distinguished by its ability to remain liquid at room temperature, making it exceptionally versatile in various scientific and industrial settings. mercury does not occur freely but is commonly found in the mineral cinnabar from which it is extracted through heating and condensing. Mercury’s applications span across multiple fields; it is used in the chemical industry as a catalyst in the production of chlorine and caustic soda via the chlor-alkali process, and in dentistry for amalgam fillings.
| Property | Value |
|---|---|
| Appearance | Silvery, liquid metal |
| Atomic Number | 80 |
| Atomic Mass | 200.592 u |
| Density at 20°C | 13.534 g/cm³ |
| Melting Point | -38.83°C (-37.89°F) |
| Boiling Point | 356.73°C (674.11°F) |
| State at 20°C | Liquid |
| Electrical Conductivity | 1.04 × 10^6 S/m |
| Thermal Conductivity | 8.30 W/(m·K) |
| Heat of Fusion | 2.29 kJ/mol |
| Heat of Vaporization | 59.11 kJ/mol |
| Specific Heat Capacity | 27.983 J/(mol·K) |
Mercury, symbolized as Hg, stands out for being the only metal liquid at room temperature and exhibits unique chemical behaviors:
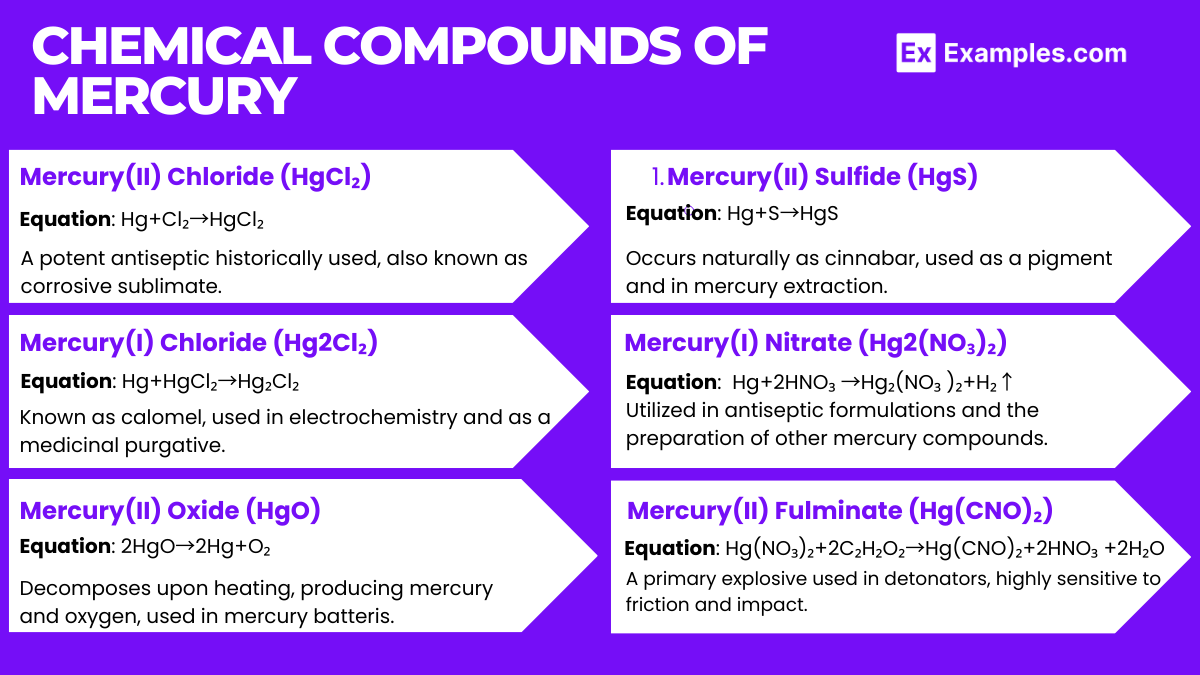
Oxidation States: Exhibits +1 (mercurous) and +2 (mercuric) oxidation states. Mercuric compounds, such as mercuric chloride (HgCl₂), are more stable and commonly encountered.
Reaction with Oxygen: Forms mercuric oxide (HgO) when heated with oxygen: 2 Hg+O₂→2 HgO₂ Hg+O₂→2 HgO
Amalgamation: Readily forms amalgams with many metals except iron, useful in metal extraction processes.
Acid Reactions: Inert to dilute acids but dissolves in nitric acid and hot concentrated sulfuric acid, forming mercuric nitrate and sulfate.
Organomercury Compounds: Forms toxic organomercury compounds, such as methylmercury (CH₃Hg⁺CH₃Hg⁺), highlighting environmental and health risks.
Volatility: Mercury vapor is highly toxic, requiring careful handling and ventilation.
| Property | Value |
|---|---|
| Melting Point | -38.83°C (-37.89°F) |
| Boiling Point | 356.73°C (674.11°F) |
| Heat of Fusion | 2.29 kJ/mol |
| Heat of Vaporization | 59.11 kJ/mol |
| Specific Heat Capacity | 27.983 J/(mol·K) |
| Thermal Conductivity | 8.34 W/(m·K) |
| Property | Value |
|---|---|
| State at Room Temperature | Liquid |
| Density | 13.534 g/cm³ at 20°C |
| Molar Volume | 14.09 cm³/mol |
| Young’s Modulus | N/A (Liquid State) |
| Shear Modulus | N/A (Liquid State) |
| Bulk Modulus | 25 GPa |
| Property | Value |
|---|---|
| Electrical Conductivity | 1.04×10^6 S/m |
| Electrical Resistivity | 96 nΩ·m (at 0°C) |
| Magnetic Susceptibility | -28.0·10^-6 cm^3/mol |
| Superconducting Point | 4.15 K |
| Property | Value |
|---|---|
| Natural Isotopes | ^196Hg, ^198Hg, ^199Hg, ^200Hg, ^201Hg, ^202Hg, ^204Hg |
| Most Stable Isotope | ^202Hg (Half-life: >1.4×10^21 years) |
| Neutron Cross Section | 374.8 barns (for ^196Hg) |
| Neutron Mass Absorption | 0.056 (for ^196Hg) |
The preparation of mercury, a unique metal that is liquid at room temperature, primarily involves the extraction and refinement from its most common ore, cinnabar (mercury sulfide, HgS). Here’s a concise overview of the process:
Below is a table highlighting some of the notable isotopes of mercury, including both stable and radioactive varieties:
| Isotope | Half-Life | Notes |
|---|---|---|
| ¹⁹⁶Hg | Stable | One of the most common stable isotopes, used in research. |
| ¹⁹⁸Hg | Stable | Also stable, present in the environment. |
| ¹⁹⁹Hg | Stable | Frequently used in NMR spectroscopy due to its nuclear spin. |
| ²⁰⁰Hg | Stable | Another stable isotope, contributing to mercury’s natural abundance. |
| ²⁰¹Hg | Stable | Used in atomic clocks and for studying brain function via tracing techniques. |
| ²⁰²Hg | Stable | The most abundant mercury isotope found in nature. |
| ²⁰⁴Hg | Stable | The least abundant stable mercury isotope. |
| ¹⁹⁴Hg | 444 years | A beta-emitter, used in research. |
| ²⁰³Hg | 46.612 days | Emits beta and gamma radiation, used in industrial and medical applications. |
Mercury’s unique properties make it invaluable across various industries and applications:
Thermometers and Barometers: Due to its liquid state at room temperature and high coefficient of expansion, mercury is used in precise thermometers, barometers, and manometers.
Dental Amalgams: Mercury’s ability to form amalgams with other metals makes it an essential component in dental fillings, providing durability and ease of application.
Electrical Switches: Mercury’s conductivity and liquid state are exploited in silent, non-sparking electrical switches, relays, and other devices where a stable, durable conductor is needed.
Chlor-alkali Process: Used in the production of chlorine and sodium hydroxide (caustic soda) through the electrolysis of brine, mercury acts as a catalyst, making the process more efficient.
Gold and Silver Mining: Mercury’s property of forming amalgams with gold and silver allows for the efficient extraction of these metals from their ores.
Preservatives and Lamps: Mercury compounds serve as preservatives in some paints and cosmetics. Mercury vapor lamps, including fluorescent lamps, use mercury to produce light, offering energy efficiency and long life.
Scientific Research: Due to its nuclear properties, mercury isotopes are used in scientific research, including nuclear physics experiments and as tracers in environmental and biological studies.
Mercury’s unique physical and chemical properties make it a fascinating element with a range of applications, from industrial to scientific. Despite its versatility, the toxic nature of mercury and its compounds calls for cautious handling and responsible environmental management. Understanding mercury’s properties and preparation underscores its significance while highlighting the need for safety and sustainability in its use
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical symbol for Mercury?
Mg
Hg
H
Mn
What is the atomic number of Mercury?
79
80
81
82
At room temperature, Mercury is in which state of matter?
Solid
Liquid
Gas
Plasma
What is the most common ore of Mercury?
Galena
Cinnabar
Sphalerite
Hematite
Mercury forms amalgams with which type of elements?
Nonmetals
Metalloids
Metals
Noble gases
Which of the following is a health concern associated with Mercury exposure?
Bone fracture
Muscle hypertrophy
Neurotoxicity
Hair loss
Which of the following is a use of Mercury in the industrial sector?
Fertilizer production
Chlor-alkali process
Textile manufacturing
Cement production
Which of the following compounds is used as a preservative in vaccines and contains Mercury?
Thimerosal
Formaldehyde
Ethanol
Benzalkonium chloride
What is the boiling point of Mercury?
356.7°C
500°C
250°C
100°C
Which type of Mercury is the most toxic to humans?
Elemental Mercury
Methylmercury
Mercurous chloride
Mercuric oxide
Before you leave, take our quick quiz to enhance your learning!

