What is the atomic number of Neptunium?
91
92
93
94
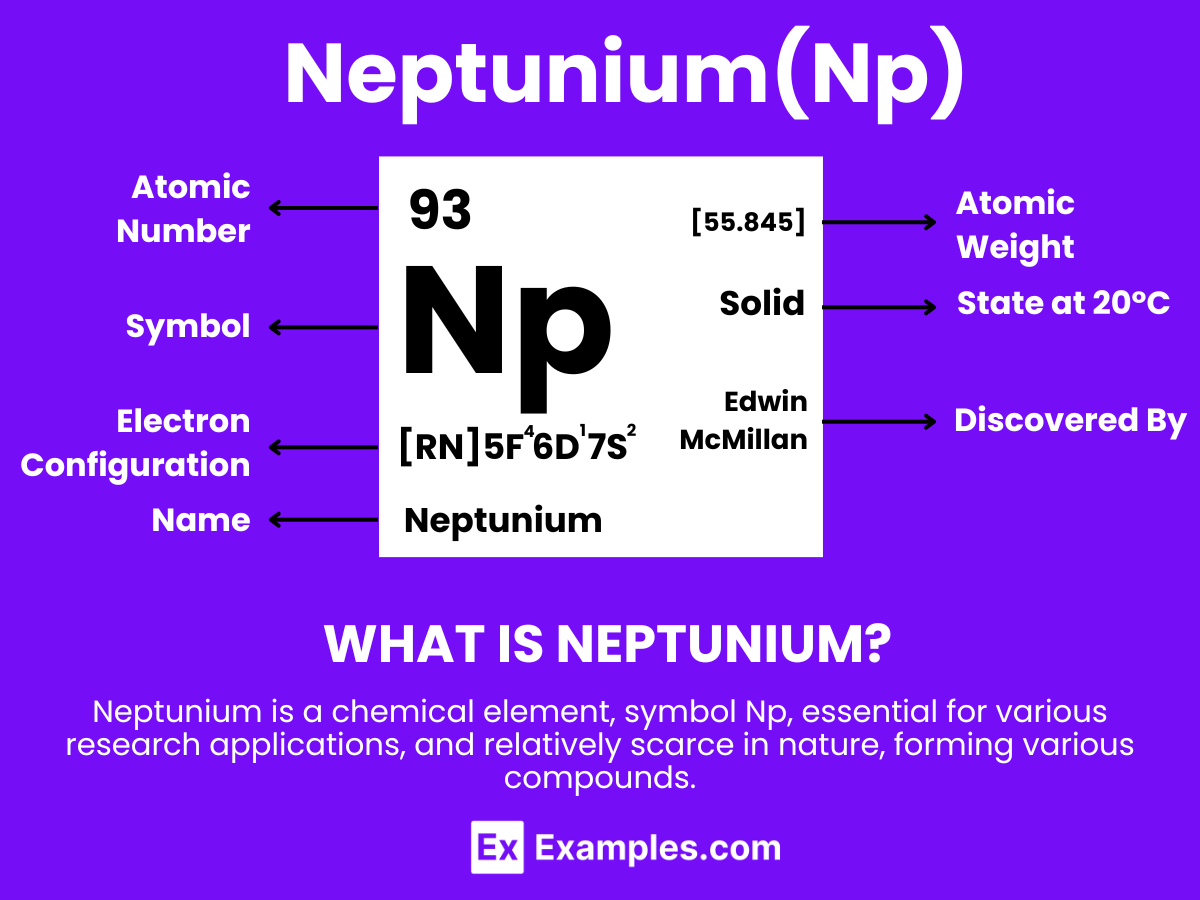
Embark on a journey through the atomic realm with our comprehensive guide to Neptunium, a transuranic and enigmatic element that bridges the gap between the known and the unknown in the periodic table. sheds light on Neptunium’s discovery, its intriguing physical and chemical properties, and its role in scientific advancements and nuclear technology. Through engaging examples, we unveil the complexities and opportunities Neptunium presents to researchers, educators, and enthusiasts alike, inviting exploration into its profound implications for future innovations.
Neptunium is a dense, silvery metallic element that is recognized for its significant properties and specialized applications. With the atomic number 93, Neptunium is notable for being the first transuranic element, marking its position in the actinide series with properties that include radioactivity and the ability to form various chemical compounds. Neptunium is primarily used in research and potential applications in nuclear science, particularly in the development of neutron detection equipment and as a precursor for plutonium-238 production, which is used in radioisotope thermoelectric generators (RTGs) for spacecraft.
| Actinium | Berkelium |
| Thorium | Californium |
| Protactinium | Einsteinium |
| Uranium | Curium |
| Plutonium | Mendelevium |
| Plutonium | Nobelium |
| Americium | Lawrencium |
| Property | Value |
|---|---|
| Appearance | Silvery, metallic, with a pink tinge |
| Atomic Mass | 237 u (most stable isotope, Np-237) |
| Density | 20.45 g/cm³ at 20 °C |
| Melting Point | 644 °C |
| Boiling Point | 4174 °C |
| State at 20 °C | Solid |
| Thermal Conductivity | 6.3 W/(m·K) |
| Crystal Structure | Orthorhombic (at room temperature) |
| Electrical Resistivity | 1.220 microohm-meters (at 22 °C) |
| Magnetic Ordering | Paramagnetic at 300 K |
Neptunium is a highly reactive, radioactive metal with a variety of oxidation states, ranging from +3 to +7, which allows it to form a diverse array of compounds. Its chemical behavior is similar to other actinides.
| Property | Value |
|---|---|
| Melting Point | 644 °C |
| Boiling Point | 4,017 °C |
| Heat of Fusion | 3.20 kJ/mol |
| Heat of Vaporization | 336 kJ/mol |
| Specific Heat Capacity | 29.46 J/(mol·K) (at 25 °C) |
| Property | Value |
|---|---|
| Density (at room temperature) | 20.45 g/cm³ |
| Crystal Structure | Orthorhombic (at room temperature) |
| Hardness | Vickers: 320 HV |
| Thermal Conductivity | 6.3 W/(m·K) |
| Electrical Resistivity | 1.220 μΩ·m (at 0 °C) |
| Property | Value |
|---|---|
| Magnetic Ordering | Paramagnetic |
| Electrical Conductivity | Metallic conductor |
| Magnetic Susceptibility | +2800.0·10⁻⁶ cm³/mol (at 298 K) |
| Property | Value |
|---|---|
| Primary Isotopes | Neptunium-237 |
| Half-life of Np-237 | 2.14 million years |
| Decay Mode of Np-237 | Alpha decay to Protactinium-233 |
| Neutron Cross Section (Np-237) | 175 barns for thermal neutrons |
| Fission Products | Various, depending on neutron capture and fission process |
| Isotope | Mass Number | Half-life | Decay Mode |
|---|---|---|---|
| Np-237 | 237 | 2.14 million years | Alpha decay |
| Np-236 | 236 | 154,000 years | Alpha decay, Neutron emission |
| Np-235 | 235 | 396.1 days | Beta decay |
| Np-239 | 239 | 2.356 days | Beta decay |
Neptunium, a synthetic element with the atomic number 93, is primarily produced in nuclear reactors. The most common isotope of neptunium, neptunium-237 (Np-237), is created through a series of nuclear reactions that begin with the absorption of a neutron by uranium-235 (U-235), one of the common fuels used in nuclear reactors. This process involves several beta decays, where a neutron within the nucleus of an atom decays into a proton, releasing electrons and antineutrinos in the process.
The production of neptunium in a nuclear reactor typically follows this sequence:
Neptunium can also be produced by bombarding uranium-238 (U-238) with neutrons in a reactor, which transforms it into plutonium-239 (Pu-239) through a series of neutron captures and beta decays. Pu-239 can then capture another neutron and undergo beta decay to become Np-239, which has a relatively short half-life and decays into plutonium-239, a process used for producing plutonium in breeder reactors.
Neptunium’s applications are limited due to its radioactivity and the complexity of handling it safely. However, it has several important uses:
Neptunium, with its unique position on the periodic table, offers a fascinating glimpse into the complex world of transuranic elements. Despite its relative obscurity and challenges in handling due to its radioactivity, its potential in scientific research and nuclear applications cannot be understated. The exploration of Neptunium not only deepens our understanding of atomic structure and nuclear physics but also paves the way for advancements in energy production and material science.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Neptunium?
91
92
93
94
What is the chemical symbol for Neptunium?
Np
Ne
Nu
Nt
Neptunium belongs to which series in the periodic table?
Lanthanides
Actinides
Transition metals
Halogens
What is the most stable isotope of Neptunium?
Neptunium-235
Neptunium-236
Neptunium-237
Neptunium-238
Neptunium was the first synthetic element discovered after which element?
Uranium
Thorium
Plutonium
Curium
In which year was Neptunium first synthesized?
1934
1936
1940
1942
Which element is formed as a decay product of Neptunium-237?
Uranium-235
Plutonium-237
Thorium-232
Protactinium-233
Neptunium is typically found in which oxidation state in aqueous solutions?
+2
+3
+4
+5
Which of the following is a common application of Neptunium?
Fuel for nuclear reactors
Fuel for nuclear reactors
Spacecraft shielding
Dental fillings
What is the primary source of Neptunium?
Natural uranium deposits
Synthesis in particle accelerators
Byproduct of nuclear reactors
Extraction from seawater
Before you leave, take our quick quiz to enhance your learning!

