What is the atomic number of Nickel?
26
27
28
29
Discover the dynamic world of Nickel, a versatile element pivotal to our modern lifestyle. From its role in crafting durable stainless steel to powering rechargeable batteries, nickel’s unique properties make it indispensable across industries. This guide delves into nickel’s journey from extraction to application, showcasing examples that highlight its significance in corrosion resistance, energy storage, and green technologies. Embark on an exploration of how nickel’s applications are shaping a sustainable future, illustrating its critical role in innovation and environmental stewardship
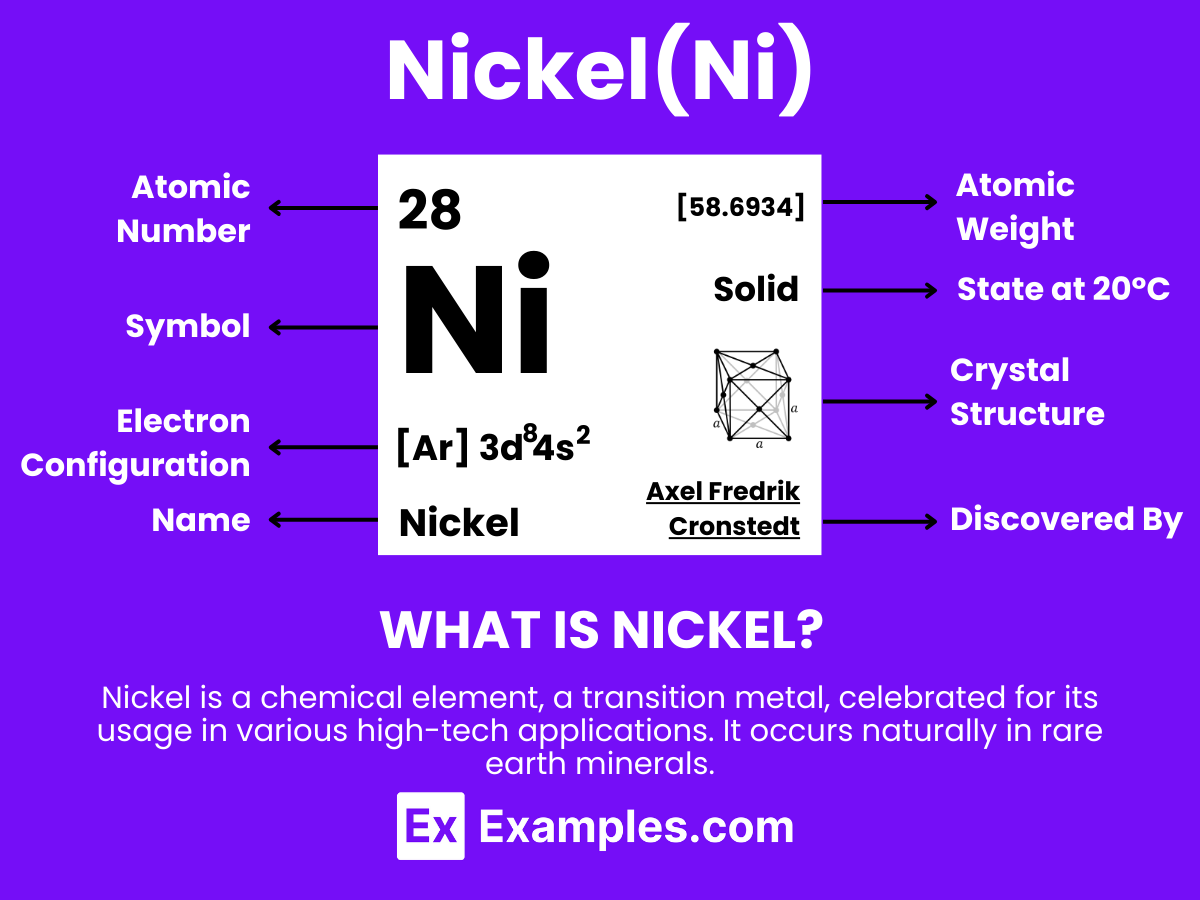
Nickel is a hard, silvery-white metallic element known for its outstanding properties and broad range of applications, with the atomic number 28. It is distinguished by its excellent resistance to corrosion and oxidation, making it an ideal material for use in extreme conditions. Nickel does not occur freely in nature but is primarily found in combination with sulfur, iron, and other metals in various ores from which it is extracted. This element is extensively utilized across numerous sectors, particularly in the production of stainless steel and other corrosion-resistant alloys used in construction, the automotive industry, and for making coins. Additionally, nickel finds important applications in the chemical industry as a catalyst for hydrogenation, in battery production, particularly in rechargeable nickel-cadmium batteries and nickel-metal hydride batteries used in portable devices and electric vehicles, and in electronics for creating durable and reliable components.
Below is a table detailing the physical properties of nickel, a highly versatile and widely utilized metallic element:
| Property | Value |
|---|---|
| Appearance | Lustrous, metallic, silver-white |
| Atomic Number | 28 |
| Atomic Mass | 58.6934 u |
| Density at 20°C | 8.908 g/cm³ |
| Melting Point | 1455°C (2651°F) |
| Boiling Point | 2913°C (5275°F) |
| State at 20°C | Solid |
| Electrical Conductivity | 14.3 × 10^6 S/m |
| Thermal Conductivity | 90.9 W/(m·K) |
| Heat of Fusion | 17.48 kJ/mol |
| Heat of Vaporization | 377.5 kJ/mol |
| Specific Heat Capacity | 26.07 J/(mol·K) |
Thermal Stability: Nickel compounds are thermally stable, suitable for high-temperature applications.
Catalytic Properties: Acts as a catalyst in hydrogenation processes, aiding in the conversion of unsaturated compounds.
Magnetic Properties: Exhibits ferromagnetic properties, useful in magnetic materials.
Environmental and Biological Role: Essential in small quantities for plant growth but toxic in high concentrations.
| Property | Value |
|---|---|
| Melting Point | 1455°C (2651°F) |
| Boiling Point | 2913°C (5275°F) |
| Heat of Fusion | 17.48 kJ/mol |
| Heat of Vaporization | 377.5 kJ/mol |
| Specific Heat Capacity | 26.07 J/(mol·K) |
| Thermal Conductivity | 90.9 W/(m·K) |
| Thermal Expansion | 13.4 µm/(m·K) at 25°C |
| Property | Value |
|---|---|
| Atomic Mass | 58.6934 u |
| Density | 8.908 g/cm³ at 20°C |
| Mohs Hardness | 4 |
| Young’s Modulus | 200 GPa |
| Bulk Modulus | 180 GPa |
| Poisson’s Ratio | 0.31 |
| Property | Value |
|---|---|
| Electrical Resistivity | 6.99 µΩ·m at 20°C |
| Magnetic Ordering | Ferromagnetic |
| Curie Temperature | 358°C (676°F) |
| Magnetic Susceptibility | High |
| Property | Value |
|---|---|
| Isotopes | Mainly ^58Ni, ^60Ni, ^61Ni, ^62Ni, ^64Ni |
| Atomic Number | 28 |
| Atomic Weight | 58.6934 |
| Stable Isotopes | ^58Ni (most abundant) |
| Neutron Cross Section | 4.5 barns (for ^58Ni) |
| Neutron Mass Absorption | 0.0012 |
The preparation of nickel involves several complex processes designed to extract and purify nickel from its ores. Nickel is predominantly obtained from two types of ore deposits: laterites and sulfides. Each type of ore requires a different extraction and processing technique. Here’s an overview of the main methods used in the preparation of nickel:
| Isotope | Mass Number | Natural Abundance (%) | Half-Life | Notes |
|---|---|---|---|---|
| Ni-58 | 58 | 68.077% | Stable | Most abundant isotope |
| Ni-60 | 60 | 26.223% | Stable | |
| Ni-61 | 61 | 1.140% | Stable | |
| Ni-62 | 62 | 3.635% | Stable | Has the highest binding energy per nucleon of any isotope |
| Ni-64 | 64 | 0.925% | Stable | |
| Ni-59 | 59 | Synthetic | 76,000 years | Used in nuclear reactors |
| Ni-63 | 63 | Synthetic | 100.1 years | Used in betavoltaic devices |
Nickel is utilized across various industries, highlighting its versatility and significance in both everyday and specialized applications.
nickel has illuminated its indispensable role across numerous industries, from high-performance alloys to green energy solutions. The detailed table we’ve presented showcases nickel’s unique physical and chemical properties, underlining its versatility and durability. This insight into nickel’s preparation and applications emphasizes its pivotal contribution to technological advancements and sustainable development, making it a key element in our pursuit of innovation
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Nickel?
26
27
28
29
Nickel is primarily obtained from which mineral?
Hematite
Bauxite
Chalcopyrite
Pentlandite
What is the symbol for Nickel on the periodic table?
Ni
Nk
Nc
Nl
Nickel belongs to which group in the periodic table?
Group 9
Group 10
Group 11
Group 12
What is the common oxidation state of Nickel?
+1
+2
+3
+4
Which of the following is a common use for Nickel?
Construction of buildings
Manufacturing of batteries
Food preservation
Production of fertilizers
Which process is commonly used to extract Nickel from its ores?
Froth flotation
Smelting
Bayer process
Electrorefining
What is the electron configuration of Nickel?
[Ar] 3d8 4s2
[Ar] 3d9 4s1
[Ar] 3d10
[Ar] 3d6 4s2
Which of the following properties is NOT associated with Nickel?
High corrosion resistance
Ferromagnetic
High electrical conductivity
Low melting point
Nickel can form alloys with many metals. Which of the following is NOT a Nickel alloy?
Monel
Invar
Bronze
Hastelloy
Before you leave, take our quick quiz to enhance your learning!

