What is the primary function of nitrogenase?
Carbon fixation
Oxygen transport
Nitrogen fixation
Protein synthesis


Nitrogenase is a remarkable enzyme that plays a crucial role in the chemistry of life. This enzyme enables bacteria to convert nitrogen from the atmosphere into complex compounds that plants can use as nutrients. Often found in the root nodules of leguminous plants, nitrogenase catalyzes the conversion process known as nitrogen fixation. This chemical reaction is vital because it helps in the production of amino acids and proteins, essential components for plant growth and survival.
Nitrogenase, the enzyme responsible for converting atmospheric nitrogen to ammonia, does not have a simple molecular formula like typical chemical compounds due to its complex protein structure. It primarily consists of two proteins: the iron protein (Fe protein) and the molybdenum-iron protein (MoFe protein). The Fe protein, also known as dinitrogenase reductase, has the formula Fe₄S₄, featuring an iron-sulfur cluster. The MoFe protein, also known as dinitrogenase, contains a unique iron-molybdenum cofactor (FeMoco), which can be represented as MoFe₇S₉C-homocitrate. This cofactor is key for the enzyme’s ability to convert nitrogen to ammonia, showcasing nitrogenase’s complexity.
Nitrogenase enzymes are classified into three main types, each distinguished by the metal cofactors present in their structure. These types are crucial for the process of nitrogen fixation in various microorganisms.
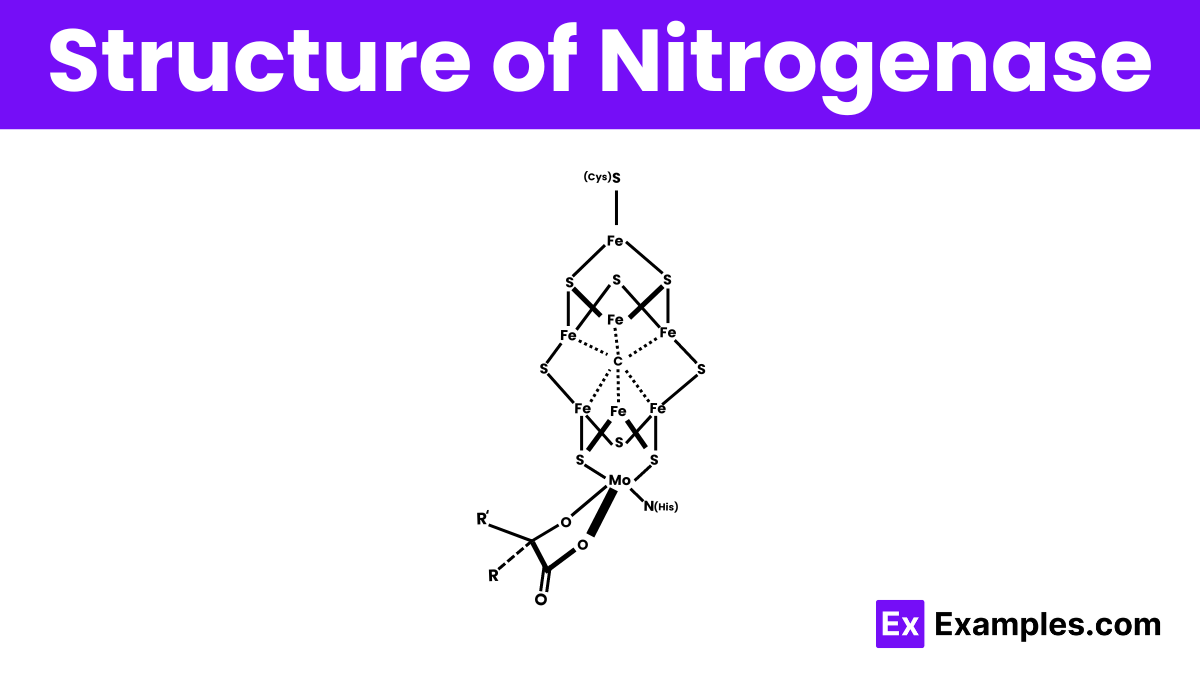
The structure of nitrogenase is complex and consists of two main protein components: the iron (Fe) protein and the molybdenum-iron (MoFe) protein. The Fe protein acts as a catalyst, providing electrons to the MoFe protein through a series of reactions. The MoFe protein, the core of the enzyme, contains a unique metal cluster known as the iron-molybdenum cofactor (FeMoco), which is where the actual nitrogen fixation reaction occurs. This cluster includes atoms of iron, molybdenum, and sulfur, arranged in a way that allows the enzyme to break down molecular nitrogen (N₂) and convert it into ammonia (NH₃), a form usable by living organisms.
Nitrogenase plays a critical role in the nitrogen cycle. An essential ecological process that converts inert atmospheric nitrogen (N₂) into ammonia (NH₃), which is then used to synthesize amino acids, proteins, and other vital organic compounds. This enzyme, primarily found in certain bacteria and archaea, enables these microorganisms to fix atmospheric nitrogen, thus fertilizing the soil naturally. Plants, unable to fix nitrogen themselves, rely on this fixed form of nitrogen for growth. As such, nitrogenase is not only pivotal for agriculture and maintaining fertile soil but also supports the base of the food chain. Through this process, nitrogenase facilitates the biological availability of nitrogen, making it crucial for life on Earth.

The primary function of nitrogenase is to convert atmospheric nitrogen (N₂) into ammonia (NH₃). This conversion is vital as it makes nitrogen available in a form that plants and other organisms can use to synthesize amino acids, nucleic acids, and other nitrogen-containing compounds.
By enabling nitrogen fixation in soil, nitrogenase helps increase soil fertility. This is especially significant in agricultural settings where leguminous plants, hosting nitrogen-fixing bacteria in their roots, enrich the soil, reducing the need for synthetic fertilizers.
Nitrogenase activity supports biodiversity by facilitating the growth of various plant species, which in turn support diverse animal populations. This enzyme’s role in nitrogen fixation helps maintain ecological balance and supports food webs.
Some nitrogen-fixing bacteria equipped with nitrogenase can also be used in bioremediation to restore nutrient levels in polluted soils. It helping to degrade organic contaminants and stabilize environments.
Nitrogenase is highly sensitive to oxygen, which can irreversibly damage its activity and structure.
Nitrogenase is important for converting atmospheric nitrogen into ammonia, facilitating plant growth and ecosystem sustainability.
Yes, particularly those involved in symbiotic relationships with plants, possess nitrogenase for nitrogen fixation.
Yes, nitrogenase reduces atmospheric nitrogen (N₂) to ammonia (NH₃), a more chemically reactive form usable by organisms.
Nitrogenase converts atmospheric nitrogen into ammonia, which is essential for synthesizing vital organic compounds in nature.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the primary function of nitrogenase?
Carbon fixation
Oxygen transport
Nitrogen fixation
Protein synthesis
Which organisms primarily possess nitrogenase?
Plants
Animals
Bacteria and archaea
Fungi
Nitrogenase activity requires which metal cofactors?
Iron and magnesium
Copper and zinc
Molybdenum and iron
Nickel and cobalt
Which molecule is a byproduct of the nitrogenase-catalyzed reaction?
Oxygen
Carbon dioxide
Hydrogen
Methane
What is the energy source for the nitrogenase enzyme?
ATP
NADH
FADH2
GTP
In which cellular compartment does nitrogenase function in bacteria?
Nucleus
Cytoplasm
Mitochondria
Plasma membrane
Which component of nitrogenase directly interacts with nitrogen gas?
P cluster
FeMo-cofactor
ATP-binding site
Hemoglobin
Which environmental condition inhibits nitrogenase activity?
Low pH
High temperature
Presence of oxygen
High salinity
Nitrogenase is crucial for which type of symbiotic relationship?
Parasitism
Mutualism
Commensalism
Competition
How many molecules of ATP are consumed per molecule of nitrogen gas fixed by nitrogenase?
2
8
16
32
Before you leave, take our quick quiz to enhance your learning!

