What is the atomic number of Oganesson?
116
117
118
119
Dive into the enigmatic world of Oganesson, a synthetic chemical marvel that sits at the pinnacle of the periodic table. This complete guide demystifies the essence of Oganesson, providing insightful examples that illuminate its properties, uses, and the fascinating science behind its creation. Uncover the intriguing role of Oganesson in advancing chemical research and its potential implications for future scientific breakthroughs. Engage with a keyword-rich narrative designed for optimal SEO and NLP compatibility, ensuring a comprehensive understanding of this extraordinary element.
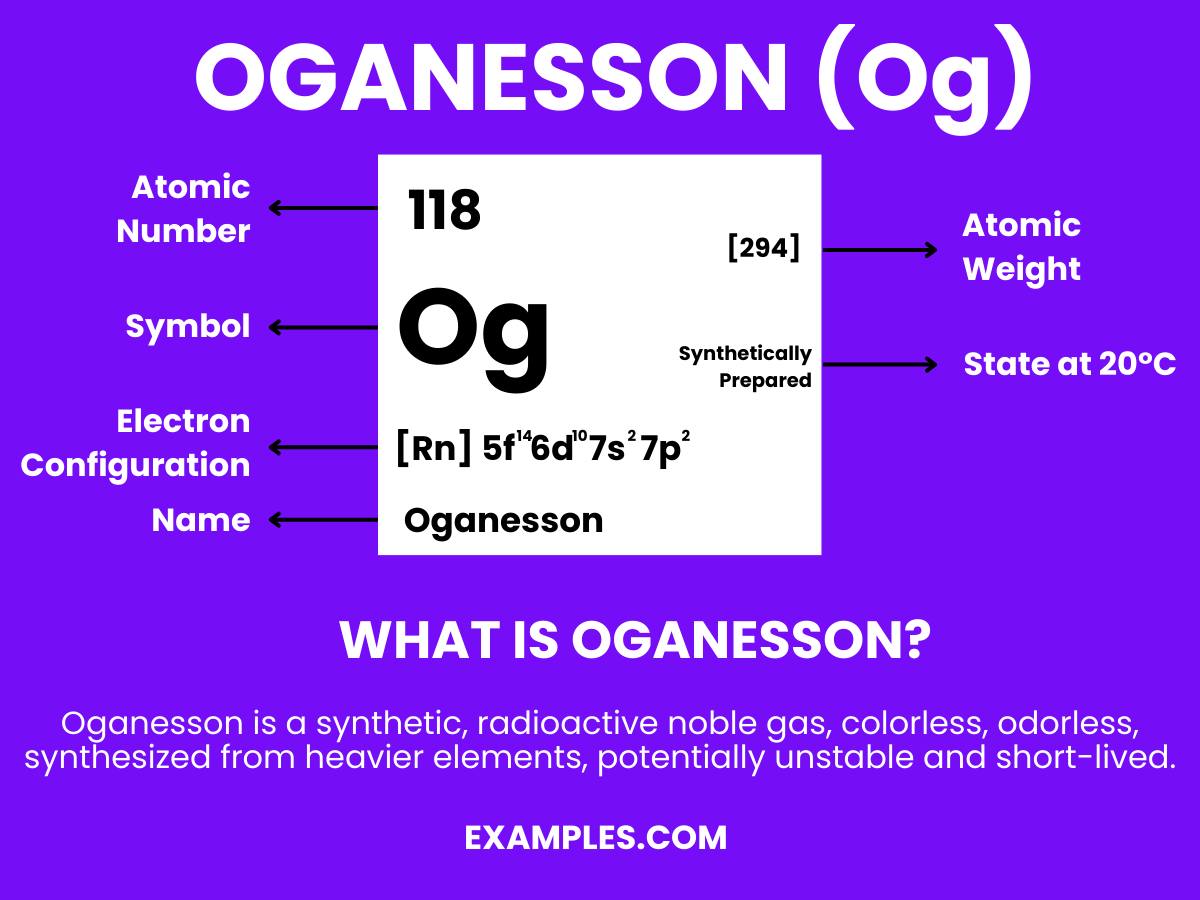
Oganesson is a superheavy, synthetic element with the chemical symbol Og and atomic number 118. It is known for being produced in particle accelerators through the fusion of atomic nuclei. Oganesson does not occur in nature and has a very short lifespan before it decays, which presents challenges for its study. The element’s discovery is crucial for nuclear physics research, especially in probing the properties and behaviors of superheavy elements in the periodic table. Because of its significant instability and radioactivity, oganesson has no practical applications beyond scientific inquiry, where it plays a role in investigating the conjectural “island of stability” and the boundaries of the periodic table.
Oganesson, a synthetic element that stands out significantly from lighter, more commonly encountered elements such as hydrogen or gallium, is a superheavy element that occupies a unique position in nuclear chemistry due to its placement in the periodic table and its categorization as a noble gas in the post-actinide series.
Atomic Level: Each atom of Oganesson (Og) is characterized by having 118 protons in its nucleus, defining its atomic number as 118. The theoretical electron configuration of Oganesson is [Rn]5f¹⁴ 6d¹⁰ 7s² 7p⁶, indicating it has a full 5f and 6d orbital, with six electrons in its 7p orbital, setting the foundation for chemical interactions. However, relativistic effects are anticipated to significantly influence its actual electron configuration, potentially altering its chemical properties.
Molecular Formation: Unlike simpler elements that can form diatomic molecules (such as H₂), Oganesson does not naturally form molecules or exhibit a stable molecular structure due to its extremely short half-life and high instability. The element exists for only milliseconds before decaying into lighter elements, making the study of its bonding characteristics and molecular formation largely theoretical. In the hypothetical scenario where Oganesson atoms could persist long enough to interact chemically, their behavior would likely be influenced by their electron configuration, but this remains speculative.
The stability and phase of Oganesson under various temperatures and pressures are subjects of theoretical speculation, as its brief existence precludes the observation of solid, liquid, or gaseous states under normal conditions. The term “Oganesson Gas” does not apply in the same way it might for compounds like uranium hexafluoride (UF₆) in the context of uranium.
| Property | Description |
|---|---|
| Appearance | Not observed directly; presumed to have no stable form due to extreme radioactivity |
| Atomic Number | 118 |
| Density (at 20°C) | 13.65 g·cm³ |
| Melting Point | Unknown; expected to be high based on periodic trends (theoretical) |
| Boiling Point | Unknown; predicted to be high, specific values not estimated (theoretical) |
| State at Room Temperature | Expected to be solid (based on theoretical calculations) |
| Electron Configuration | [Rn] 5f¹⁴ 6d¹⁰ 7s² 7p⁶ |
| Common Oxidation States | +2, +4 (predicted, but not co |
Oganesson, with the atomic number 118, is a synthetic element located in group 18 of the periodic table.
Investigating the chemical properties of Oganesson remains a largely speculative endeavor, dependent on future advancements in experimental methods and the synthesis of more stable isotopes for comprehensive analysis.
| Property | Value with Unit |
|---|---|
| Atomic Number | 118 |
| Atomic Mass | Most stable isotope: Oganesson-294 (294 u) |
| Isotopes | Various, including ^294Og (predicted to be the most stable) |
| Half-Life (for ^294Og) | Less than a millisecond (estimated) |
| Nuclear Spin | Not precisely determined due to short half-lives |
| Neutron Cross Section | Not determined (extremely short-lived isotopes make measurement challenging) |
Oganesson is a superheavy, synthetic element that does not occur naturally and can only be synthesized in a laboratory setting. The preparation of oganesson involves highly specialized equipment, including advanced nuclear reactors and ion accelerators. Here is an outline of the general process used to create oganesson:
Selection of Target and Projectile:
Acceleration:
Collision and Fusion:
Nucleus Cooling and Decay:
Detection and Identification:
Isolation of Isotopes:
A theoretical compound suggesting the interaction between oganesson and oxygen to form an oxide.
Equation: 2Og + O₂ → OgO₂
Predicts the formation of a fluoride compound when oganesson reacts with fluorine.
Equation: 2Og + F₂ → OgF₂
Suggests the possibility of oganesson combining with chlorine to form a chloride compound.
Equation: 2Og + Cl₂ → OgCl₂
Indicates the theoretica₂ l reaction between oganesson and bromine to produce a bromide.
Equation: 2Og + Br₂ → OgBr₂
Inference about oganesson’s ability to react with iodine to form an iodide compound.
Equation: 2Og + I₂ → OgI₂
Speculates on the reaction between oganesson and hydrogen to create a hydride.
Equation: 2Og + H₂ → OgH₂
Oganesson is a synthetic element with no stable isotopes. Its isotopes have been created in laboratory settings through nuclear reactions, showcasing distinct decay characteristics.
| Isotope | Half-Life | Decay Mode |
|---|---|---|
| Og-293 | Less than 0.69 ms | Alpha decay to Lv-289 |
| Og-294 | Predicted, not observed | Predicted alpha decay |
| Og-295 | Predicted, not observed | Predicted alpha decay |
| Og-296 | Predicted, not observed | Predicted alpha decay to Lv-292 |
| Og-297 | Predicted, not observed | Predicted alpha decay |
| Og-298 | Predicted, not observed | Predicted alpha decay |
| Og-299 | Predicted, not observed | Predicted alpha decay |
Oganesson, element 118, is the heaviest element currently recognized by the International Union of Pure and Applied Chemistry (IUPAC).
Oganesson, with atomic number 118, represents one of the latest frontiers in the exploration of the periodic table. Due to its position as a superheavy element and its incredibly short half-life, practical applications of Oganesson outside theoretical research are yet to be realized. However, its discovery and the ongoing investigations into its properties hold significant implications across various scientific domains:
Oganesson stands as a monumental achievement in the field of chemistry, symbolizing the zenith of human curiosity and scientific exploration. Though its practical applications remain speculative, its discovery pushes the boundaries of the periodic table and enhances our understanding of the atomic world. Oganesson’s study promises to inspire future scientific breakthroughs, underscoring the endless pursuit of knowledge.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Oganesson?
116
117
118
119
What is the chemical symbol for Oganesson?
Og
Os
Ogm
Oa
Oganesson belongs to which group in the periodic table?
Group 16
Group 17
Group 18
Group 19
Which period does Oganesson belong to in the periodic table?
Period 6
Period 7
Period 8
Period 9
Oganesson is expected to have what type of state at room temperature?
Solid
Liquid
Gas
Plasma
Which property is unusual for Oganesson compared to lighter noble gases?
High reactivity
High density
Low melting point
Low atomic mass
Oganesson is named in honor of which scientist?
Yuri Oganessian
Dmitri Mendeleev
Glenn T. Seaborg
Marie Curie
What is the primary method used to synthesize Oganesson?
Electrolysis
Nuclear fusion reactions
Chemical vapor deposition
Distillation
Oganesson is predicted to exhibit which type of chemical behavior?
Similar to helium
Similar to krypton
Similar to radon
Unique due to relativistic effects
Which of the following is a challenge in studying Oganesson?
High abundance
Long half-life
Rapid decay
Stability in compounds
Before you leave, take our quick quiz to enhance your learning!

