What is the chemical symbol for Osmium?
Os
Om
Sm
Osu
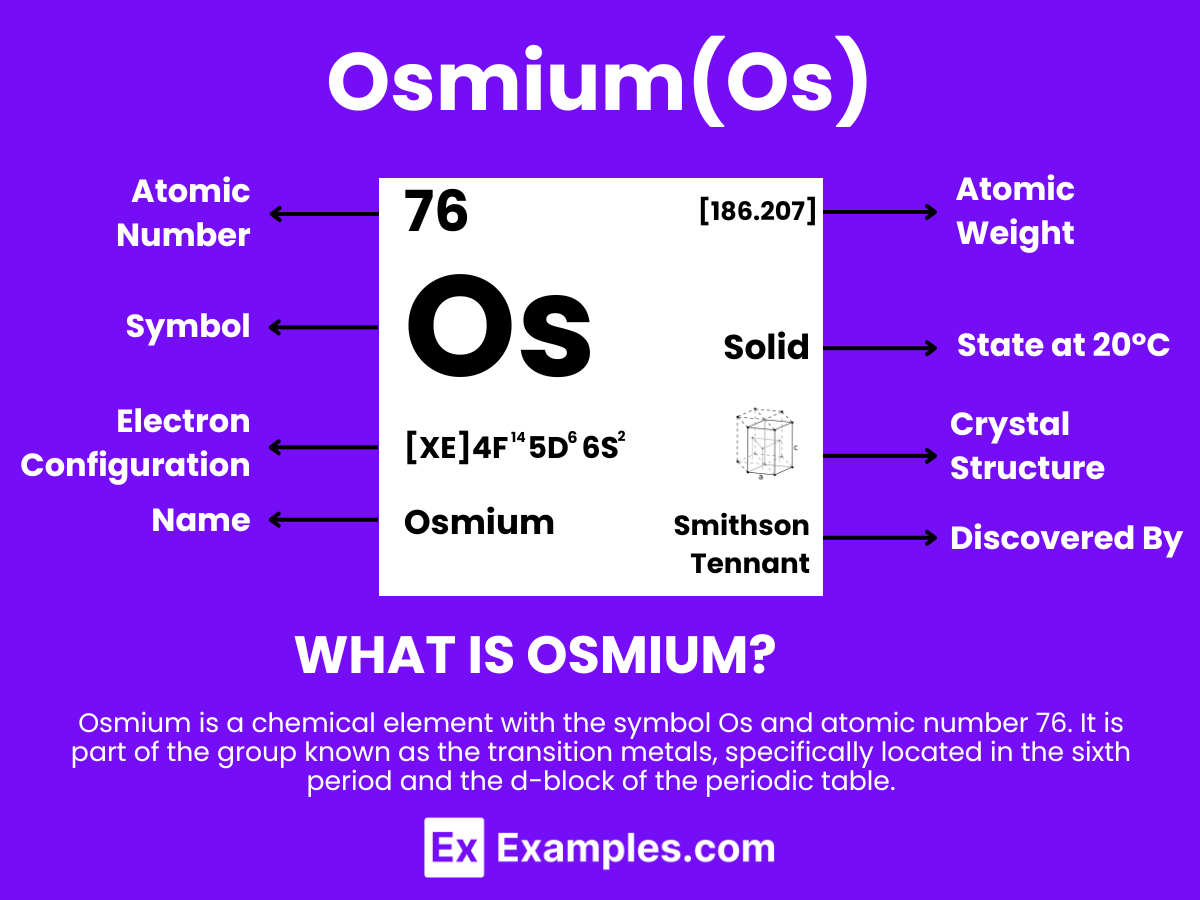
Embark on an exploration of Osmium, the heaviest and densest naturally occurring element known for its remarkable hardness and luster. This comprehensive guide unveils Osmium’s definition, delves into its myriad uses from strengthening alloys to precision instruments, and uncovers the fascinating chemistry behind its compounds. As a pivotal material in science and industry, Osmium’s unique properties not only challenge our understanding of metallic behavior but also offer innovative solutions to modern technological demands.
Osmium is a metallic element with the chemical symbol Os and atomic number 76. It is extracted from ores containing platinum metals, where it occurs in low concentrations. Osmium is one of the densest elements in the Earth’s crust and has one of the highest melting points of all elements, making it highly valued for its durability and resistance to wear and corrosion in extreme environments. The discovery of osmium was significant in the field of chemistry for its unique properties among the platinum group metals in the periodic table. Its exceptional density and hardness, along with its catalytic properties, make it crucial in applications requiring materials that can withstand extreme pressure and chemical reactions, such as in electrical contacts.
The elemental formula for Osmium is simply “Os”. This concise representation embodies a complex element characterized by its unmatched density and stability.
Delving into the atomic structure of Osmium reveals why it’s the densest element on the periodic table:
This comprehensive overview of Osmium, from its fundamental formula and atomic structure to its practical applications, highlights the element’s significant role across various scientific and industrial domains.
| Physical Property | Description |
|---|---|
| Density | 22.59 g/cm³ at 20 °C, the highest among all elements |
| Melting Point | 3033°C (5491°F) |
| Boiling Point | 5012°C (9054°F) |
| State at 20 °C | Solid |
| Appearance | Bluish-white, lustrous metal |
| Crystal Structure | Hexagonal close-packed (hcp) |
| Thermal Conductivity | 87.6 W/(m·K) (at 300 K) |
| Electrical Conductivity | Lower than most metals but sufficient for some applications |
Osmium, with the symbol Os and atomic number 76, is a hard, brittle, bluish-white transition metal in the platinum group, known for its high density and remarkable stability. It exhibits a wide range of oxidation states, from -2 to +8, with +4 and +8 being the most common.
| Property | Value |
|---|---|
| Melting Point | 3033 °C |
| Boiling Point | 5012 °C |
| Heat of Fusion | 31 kJ/mol |
| Heat of Vaporization | 378 kJ/mol |
| Specific Heat Capacity | 130 J/(kg·K) |
| Property | Value |
|---|---|
| Density | 22.59 g/cm³ |
| Mohs Hardness | 7 |
| Vickers Hardness | 3920 MPa |
| Bulk Modulus | 462 GPa |
| Tensile Strength | Not readily available |
| Property | Value |
|---|---|
| Electrical Resistivity | 81.2 nΩ·m |
| Thermal Conductivity | 87.6 W/(m·K) |
| Magnetic Susceptibility | -0.0002 (dimensionless) |
| Property | Value |
|---|---|
| Atomic Number | 76 |
| Atomic Mass | 190.23 u |
| Isotopes | 184, 186, 187, 188, 189, 190, 192 |
| Stable Isotopes | 184, 187, 188, 189, 190, 192 |
Osmium, with its unique and extreme properties, is both rare and challenging to prepare in pure form. Here are five key points regarding the preparation process of osmium:
Osmium tetroxide is one of the most significant compounds of osmium,
Equation: Os+4O₂→2OsO₄
Osmium dioxide is another oxide of osmium, showcasing the metal’s ability to exhibit different oxidation states.
Equation: Os+O₂→OsO₂
Potassium osmate is the product of the aqueous reaction of osmium tetroxide with potassium hydroxide.
Equation: K₂[OsO₂(OH)₄]
Osmium hexachloride emphasizes osmium’s ability to form halides, contributing to its comprehensive halogen chemistry.
Equation: Os+3Cl₂→OsCl₆
Osmium chloride is a complex ion demonstrating osmium’s ability to form coordination compounds with ammonia.
Equation:Os+6NH₃+3Cl₂
Osmium oxide hydrate is a hydrated form of osmium tetroxide.
Equation: OsO₄+nH₂O
| Isotope | Half-life | Natural Abundance |
|---|---|---|
| Os-184 | Stable | 0.02% |
| Os-187 | Stable | 1.6% |
| Os-188 | Stable | 13.3% |
| Os-189 | Stable | 16.1% |
| Os-190 | Stable | 26.4% |
| Os-192 | Stable | 41.0% |
Osmium’s unique properties, including its extreme density, hardness, and resistance to wear, make it valuable in a variety of specialized applications:
Osmium, the densest naturally occurring element, plays a pivotal role in specialized industrial and scientific applications due to its unique combination of high density, hardness, and chemical stability. Though produced in minimal quantities, its use in electrical contacts, precision instruments, and catalysis underscores its invaluable contribution to advanced technology and scientific research.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical symbol for Osmium?
Os
Om
Sm
Osu
Osmium belongs to which group in the periodic table?
Group 6
Group 7
Group 8
Group 9
What is the density of Osmium?
19.1 g/cm³
21.45 g/cm³
22.59 g/cm³
23.1 g/cm³
Which property is Osmium most known for?
High reactivity
Low melting point
High density
High electrical conductivity
Osmium is a member of which series of transition metals?
Lanthanides
Actinides
Platinum group metals
Alkali metals
Which of the following compounds is Osmium commonly found in?
Osmium tetroxide
Osmium dichloride
Osmium sulfate
Osmium fluoride
What is one of the primary uses of Osmium tetroxide?
Fuel additive
Catalyst in chemical reactions
Staining agent in microscopy
Water purification
What is the melting point of Osmium?
2000°C
2500°C
3033°C
3500°C
Which of the following is a characteristic property of Osmium?
Brittle at room temperature
High volatility
Resistant to corrosion
Soluble in water
Which acid can dissolve Osmium?
Aqua regia
Hydrochloric acid
Sulfuric acid
Nitric acid
Before you leave, take our quick quiz to enhance your learning!

