Which isotope of plutonium is most commonly used in nuclear reactors and weapons?
Pu-238
Pu-239
Pu-240
Pu-241
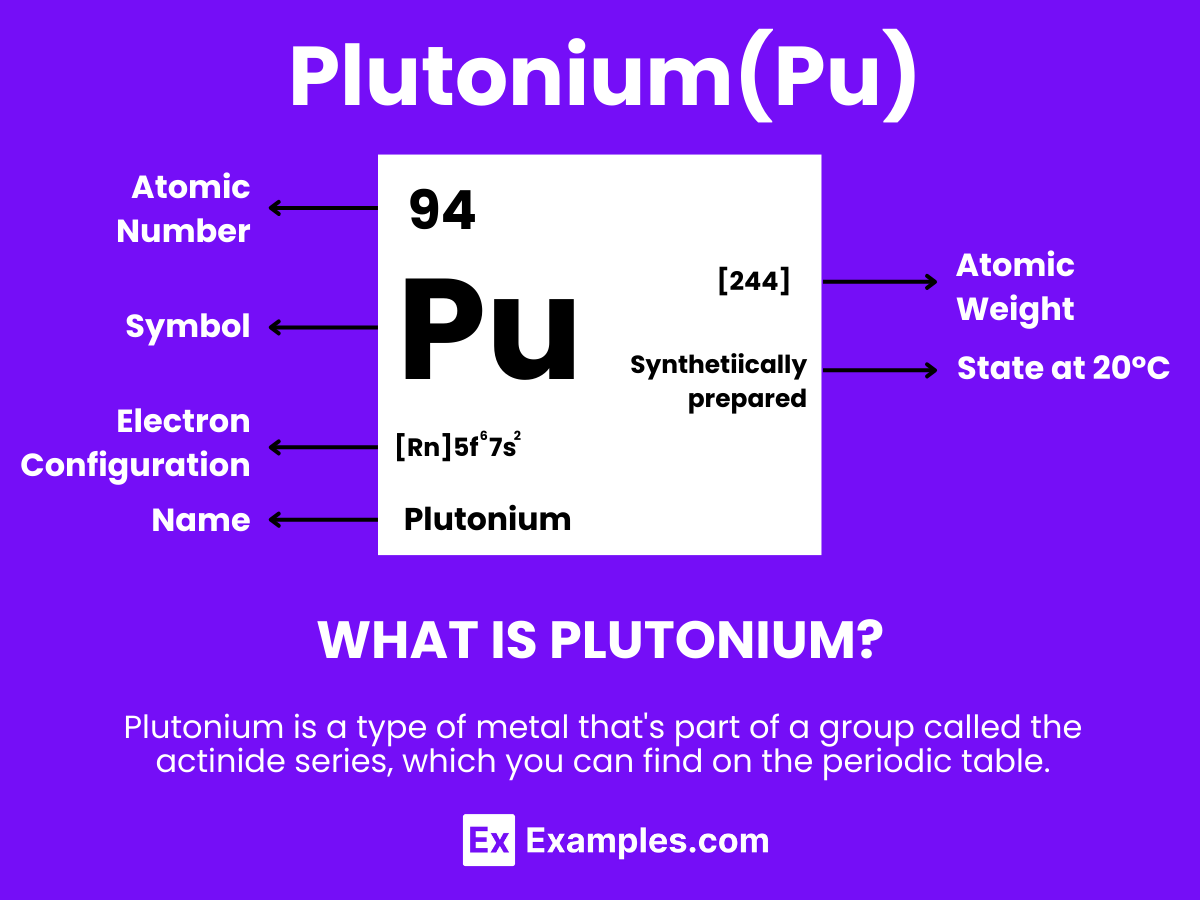
Discover the intriguing world of Plutonium, a pivotal element in the realm of nuclear science and technology. Plutonium, with its complex characteristics and applications, stands at the forefront of advancements in energy and defense. This guide delves into the fascinating aspects of Plutonium, from its discovery to its role in powering spacecraft and strengthening national security. Through real-life examples, we unveil the multifaceted uses of Plutonium, showcasing its significance in modern technology and research. Embark on a journey to explore the compelling narrative of Plutonium, a key player in shaping our understanding and utilization of nuclear energy.
| Actinium | Berkelium |
| Thorium | Californium |
| Protactinium | Einsteinium |
| Uranium | Curium |
| Neptunium | Mendelevium |
| Fermium | Nobelium |
| Americium | Lawrencium |
Plutonium is a synthetic, silvery-gray metallic element with the atomic number 94, known for its critical role in nuclear energy and weaponry due to its high radioactivity and ability to sustain a nuclear chain reaction. Unlike naturally occurring elements, Plutonium is produced in nuclear reactors by bombarding Uranium-238 with neutrons, making it pivotal in the manufacture of nuclear weapons and as a reactor fuel. Beyond its nuclear applications, Plutonium powers thermoelectric generators in spacecraft for long-duration missions. However, its radioactive nature and potential health risks necessitate strict safety and environmental protocols to manage its use and mitigate impacts on human health and the environment
| Property | Description |
|---|---|
| Appearance | Silvery-gray metallic; tarnishes to a dull gray in air |
| Atomic Number | 94 |
| Atomic Mass | 244 u |
| Density (at room temperature) | 19.86 g/cm³ |
| Melting Point | 639.4 °C (1182.9 °F) |
| Boiling Point | 3228 °C (5842.4 °F) |
| State at Room Temperature | Solid |
| Crystal Structure | Monoclinic (alpha), Face-centered cubic (delta) at higher temperatures |
| Thermal Conductivity | 6 W/(m·K) (at 25 °C) |
| Electrical Conductivity | Relatively poor conductor of electricity |
| Radioactivity | Highly radioactive, primarily alpha emitter |
Plutonium, a synthetic element with the symbol Pu and atomic number 94, exhibits a complex array of chemical properties, making it a subject of extensive study within nuclear chemistry. As a member of the actinide series, Plutonium shares characteristics with other radioactive elements, displaying multiple oxidation states and reacting with various non-metals. Its chemical behavior is not only pivotal for understanding nuclear reactions but also for the safe handling and storage of nuclear materials.
Oxidation States and Reactivity: Plutonium can exist in multiple oxidation states, ranging from +3 to +7, with +4 (PuO₂ ) and +3 (Pu₂O₃) being the most stable. This variability in oxidation states allows Plutonium to form a variety of compounds, including oxides, halides, and hydrides. For instance, Plutonium reacts with oxygen to form PuO, PuO₂ , and Pu₂O₃, with Plutonium dioxide (PuO₂) being the most significant due to its use in nuclear fuel:
2 Pu+O₂→2 PuO₂
Reaction with Acids and Halogens: Plutonium dissolves in acidic solutions, forming Pu(IV) ions, and reacts vigorously with halogens to produce halides. For example, the reaction with chlorine gas yields Plutonium tetrachloride (PuCl₄):
Pu+2 Cl₂ →PuCl₄
Hydride Formation: When exposed to hydrogen gas, Plutonium forms Plutonium hydride (PuH2+x), a reaction critical for understanding Plutonium’s behavior in nuclear storage conditions:
Pu+H₂ →PuH₂ +x
Complexation and Solubility: Plutonium’s ability to form complex ions affects its solubility and mobility in the environment, particularly in water and biological systems. Its interaction with organic and inorganic ligands can stabilize various Plutonium species, influencing its bioavailability and environmental impact.
Radioactive Decay and Transmutation: Plutonium undergoes alpha decay, transforming into other actinides and eventually leading to stable isotopes. For example, Pu-239 decays to Uranium-235, a process that is fundamental to the operation of nuclear reactors and the formation of nuclear weapons:
239Pu→235U+α
Environmental and Safety Considerations: The chemical properties of Plutonium, particularly its reactivity and radioactivity, necessitate rigorous safety protocols in its handling, storage, and disposal. The potential for environmental contamination through leaching, airborne particles, or nuclear accidents underscores the importance of understanding Plutonium’s chemical behavior.
| Property | Value |
|---|---|
| Melting Point | 639.4 °C (1182.9 °F) |
| Boiling Point | 3228 °C (5842.4 °F) |
| Heat of Fusion | 2.82 kJ/mol |
| Heat of Vaporization | 333.5 kJ/mol |
| Specific Heat Capacity (@25 °C) | 35.5 J/(mol·K) |
| Property | Value |
|---|---|
| Density (@20 °C, α-phase) | 19.86 g/cm³ |
| Thermal Conductivity | 6.74 W/(m·K) |
| Electrical Resistivity (@20 °C) | 1.460 microohm-meters |
| Young’s Modulus | 96 GPa |
| Poisson’s Ratio | 0.21 |
| Property | Value |
|---|---|
| Electrical Conductivity | Variable; dependent on allotrope and purity |
| Magnetic Ordering | Paramagnetic at room temperature (α-phase) |
| Superconductivity | Not naturally superconducting; can become superconducting under high pressure |
| Property | Value |
|---|---|
| Atomic Number | 94 |
| Isotopes | Mainly Pu-238, Pu-239, Pu-240, Pu-241 |
| Half-life of Pu-239 | 24,110 years |
| Neutron Cross Section (Pu-239) | 749 barns (for thermal neutrons) |
| Critical Mass (Pu-239) | ~10 kg (spherical configuration) |
A brown/black powder, crucial for nuclear fuel applications. Synthesis
equation: Pu+O₂→PuO₂
Green crystalline solid, used in plutonium separation processes.
Formation reaction: Pu+23Cl₂→PuCl₃
Bright yellow-green powder, essential for nuclear material processing.
Synthesized by: 4Pu+2F₂→PuF₄
A soluble compound, pivotal for plutonium extraction and purification steps.
Prepared through: Pu(OH)₄+4HNO₃→Pu(NO₃)₄+4H₂O
Less common, unstable yellow powder, used in advanced nuclear research.
Formation process: 2Pu+23O₂→Pu2O₃
A pyrophoric compound that reacts with air, used in nuclear research.
Generated via: 2Pu+3H₂→2PuH₃
| Isotope | Half-Life | Decay Mode |
|---|---|---|
| Pu-238 | 87.7 years | Alpha decay |
| Pu-239 | 24,110 years | Alpha decay |
| Pu-240 | 6,563 years | Alpha decay |
| Pu-241 | 14 years | Beta decay to Americium-241 |
| Pu-242 | 373,300 years | Alpha decay |
| Pu-244 | 80 million years | Alpha decay |
Plutonium is primarily produced in nuclear reactors through neutron irradiation of Uranium-238 (U-238), one of the most common isotopes of uranium found in nature. The process begins when U-238 absorbs a neutron and becomes Uranium-239 (U-239), which is unstable. Through beta decay, U-239 transforms into Neptunium-239 (Np-239), and subsequently, Np-239 undergoes another beta decay to become Plutonium-239 (Pu-239), a process that can be represented by the following equations:
U-238+n→U-239
This production method highlights the synthetic nature of Plutonium, as it does not occur naturally in significant amounts on Earth. The Plutonium produced can then be chemically separated from the spent nuclear fuel through various reprocessing methods, such as PUREX (Plutonium-Uranium Extraction), making it available for further use.
Plutonium’s unique properties have led to a wide range of applications, most notably in nuclear energy and weaponry.
Plutonium has illuminated its multifaceted roles in science, energy production, and beyond. This dense material, pivotal for its applications in nuclear reactors and medicine, underscores a blend of challenge and opportunity. Our journey through its properties, uses, and implications not only enriches our understanding but also propels forward-thinking in harnessing its potential resonsibly.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Which isotope of plutonium is most commonly used in nuclear reactors and weapons?
Pu-238
Pu-239
Pu-240
Pu-241
Plutonium was first discovered in which year?
1934
1940
1945
1950
What is the primary use of plutonium-238?
Nuclear weapons
Spacecraft power sources
Cancer treatment
Industrial radiography
What color does plutonium exhibit in its pure form?
Silver
Blue-gray
Red
Yellow
Which chemical property makes plutonium hazardous?
Non-reactivity
High melting point
Radioactivity
Solubility in water
Plutonium belongs to which series in the periodic table?
Lanthanides
Actinides
Transition metals
Halogens
Which process is used to produce plutonium in nuclear reactors?
Fission
Fusion
Neutron capture
Alpha decay
Plutonium has how many allotropes at room temperature?
Two
Four
Six
Seven
Plutonium dioxide (PuO2) is often used as:
A fuel in nuclear reactors
A pigment in paints
An insecticide
A lubricant
What type of radiation is primarily emitted by plutonium-239?
Alpha particles
Beta particles
Gamma rays
Neutrons
Before you leave, take our quick quiz to enhance your learning!

