What is the chemical symbol for Protactinium?
Pa
Pr
Pt
Pb
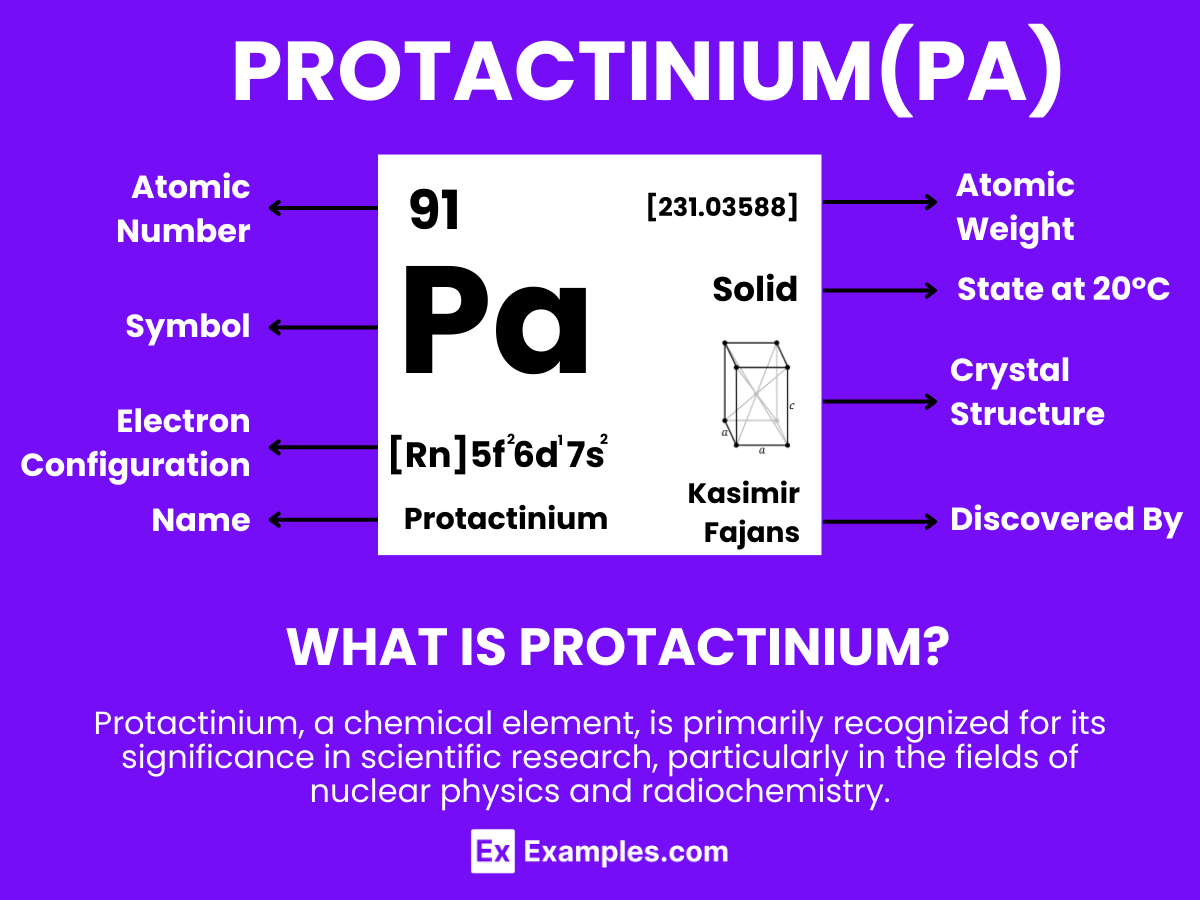
Discover the intriguing world of rare elements with our comprehensive guide on Protactinium, a lesser-known player in the periodic table. Delve into the mysteries and applications of Protactinium, from its discovery to its unique properties and uses in various fields. This guide offers insights and examples that illuminate the significance of Protactinium in scientific research and technological advancements. Whether you’re a student, researcher, or science enthusiast, explore the fascinating aspects of Protactinium and its contribution to our understanding of the atomic world.
Protactinium is a dense, silvery-gray metallic element that is characterized by its unique properties and limited applications. With the atomic number 91, Protactinium is notable for its radioactivity and scarcity in nature, making it one of the less common elements. This element does not occur freely in nature but is usually found in uranium ores from which it is extracted. Protactinium is mainly used in scientific research rather than widespread industrial applications, due to its radioactivity and scarcity. The element has been studied for potential use in nuclear reactors and as a tracer for geochronology and paleoceanography studies, but its high radiotoxicity and limited availability restrict its practical applications
The electron configuration of Protactinium is [Rn] 5f² 6d¹ 7s². This indicates that Protactinium has two electrons in the 5f orbital, one electron in the 6d orbital, and two electrons in the 7s orbital, following the filled orbitals of radon (Rn). The configuration highlights its position as an early actinide, where the filling of the 5f orbital begins.
The nucleus of a Protactinium atom comprises 91 protons, imparting a positive charge, and a variable number of neutrons across its isotopes, with ^231Pa containing 140 neutrons. The delicate balance between protons and neutrons plays a crucial role in the stability and radioactivity of Protactinium isotopes.
| Property | Value |
|---|---|
| Appearance | Bright, silvery metallic luster |
| Atomic Number | 91 |
| Atomic Weight | 231.03588 u |
| Density | 15.37 g/cm³ (near room temperature) |
| Melting Point | 1568 °C (2854 °F; 1841 K) |
| Boiling Point | 4027 °C (7281 °F; 4300 K) |
| State at 20 °C | Solid |
| Crystal Structure | Orthorhombic |
| Thermal Conductivity | ~47 W/(m·K) (at 300 K) |
| Electrical Resistivity | Not well characterized due to radioactivity |
| Thermal Expansion | Not well characterized |
| Young’s Modulus | Not well characterized |
| Shear Modulus | Not well characterized |
| Bulk Modulus | Not well characterized |
| Mohs Hardness | Approx. 3-4 |
| CAS Number | 7440-13-3 |
Protactinium (Pa) is a dense, silvery-gray metal belonging to the actinide series. With its atomic number 91, it exhibits intriguing chemical properties due to its position in the periodic table.
1. Oxidation States: Protactinium primarily exhibits the +5 oxidation state in its compounds, which is the most stable and common. However, it can also show a +4 oxidation state in some compounds.
2. Reaction with Air: Protactinium is quite reactive with oxygen. In the air, it forms protactinium oxide. For the +5 oxidation state, the reaction can be represented as: 2 Pa+5 O₂→2 PaO₅
And for the +4 state, the reaction is: 4 Pa+5 O₂→2 Pa2O₄
3. Reaction with Water: Protactinium reacts with water, but the reaction is not as vigorous as with some other actinides. The reaction forms protactinium oxide and hydrogen gas: Pa+2 H₂O→PaO₂+2 H₂
4. Reaction with Acids: Protactinium dissolves in hydrochloric acid (HCl) and nitric acid (HNO3), forming protactinium(IV) or protactinium(V) solutions depending on the conditions, and releasing hydrogen gas: Pa+4 HCl→PaCl₄+2 H₂
Pa+5 HNO₃ →Pa(NO₃ )₅+H₂
5. Compounds and Complexes:Protactinium Oxides Protactinium forms oxides in both +4 and +5 oxidation states, PaO₂ and Pa2O₅, important for understanding its chemistry.
Protactinium Halides Including fluorides, chlorides, bromides, and iodides, such as PaF₄ and PaCl₅, showcasing its ability to form complex halide compounds.
6. Behavior with Halogens: Protactinium reacts with halogens to form tetra- and penta-halides. For example, with fluorine, it forms PaF4 and PaF5, indicating its reactive nature towards halogens: Pa+2 F₂→PaF₄
Pa+5 F₂→PaF₅
7. Radioactivity: Protactinium is highly radioactive, with isotopes such as Protactinium-231 playing a crucial role in the uranium-235 decay series. Its radioactivity is a significant aspect of its chemical behavior, influencing its handling and storage.
The table below lists some of the isotopes of protactinium, including their mass numbers, half-lives, and decay modes:
| Isotope | Mass Number | Half-Life | Decay Mode |
|---|---|---|---|
| Protactinium-230 | 230 | 17.4 days | Beta decay to Uranium-230 |
| Protactinium-231 | 231 | 32,760 years | Alpha decay to Actinium-227 |
| Protactinium-232 | 232 | 1.31 days | Beta decay to Uranium-232 |
| Protactinium-233 | 233 | 26.967 days | Beta decay to Uranium-233 |
| Protactinium-234 | 234 | 6.75 hours | Beta decay to Uranium-234 |
Protactinium(IV) Chloride is a yellow crystalline solid, used in research applications. It forms through the reaction: Pa+2Cl₂→PaCl₄
Protactinium(V) Oxide is a pale yellow powder, crucial for studying Protactinium’s chemical behavior. It’s synthesized by: 2Pa+5O₂→Pa2O₅
A pale yellow crystalline solid, Protactinium(V) Fluoride is produced in the reaction: Pa+5F₂→PaF₅
Protactinium(IV) Iodide, a yellow crystalline substance, is prepared through: Pa+2I₂→PaI₄
A green-yellow crystalline solid, it’s obtained by combining Protactinium with chlorine: Pa+5Cl₂→PaCl₅
Protactinium(IV) Oxide, a black powder, is essential for understanding Protactinium’s properties. It’s formed by: 2Pa+2O₂→2PaO₂
Protactinium, due to its scarcity, radioactivity, and challenging handling requirements, has limited but specialized uses:
The production of Protactinium, a rare and highly radioactive element, is predominantly a byproduct of the nuclear industry. Its scarcity, combined with its hazardous radioactivity, makes its production complex and highly specialized. The most common isotope, Protactinium-231, originates from the decay of Uranium-235, while Protactinium-233 is produced through neutron irradiation of Thorium-232. Below are the main methods used in the production of Protactinium:
Despite its challenges, Protactinium has several niche applications, primarily in research and nuclear science:
This article has explored the intricate properties and meticulous preparation processes of Protactinium, a rare and fascinating element. Through our detailed examination, we’ve uncovered the physical and chemical characteristics that make Protactinium unique, as well as the complex methods involved in its extraction and purification. This knowledge not only enriches our understanding of Protactinium but also highlights its potential applications in science and technology.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the chemical symbol for Protactinium?
Pa
Pr
Pt
Pb
What is the atomic number of Protactinium?
91
92
93
94
Protactinium is classified under which category of elements?
Noble gases
Lanthanides
Actinides
Transition metals
In which period of the periodic table is Protactinium located?
6
7
5
4
What is the most stable isotope of Protactinium?
Protactinium-231
Protactinium-232
Protactinium-230
Protactinium-234
Protactinium is most commonly found in which type of mineral?
Limestone
Phosphate rocks
Uranium ores
Coal
Who discovered Protactinium, and in what year?
Marie Curie, 1917
Otto Hahn, 1913
Glenn T. Seaborg, 1945
Friedrich Oskar Giesel, 1898
What is a primary application of Protactinium in modern science?
Medicine
Nuclear reactors
Electronics
Catalysts
What is the density of Protactinium?
15.37 g/cm³
13.55 g/cm³
11.87 g/cm³
17.45 g/cm³
What type of radiation does Protactinium primarily emit?
Alpha particles
Beta particles
Gamma rays
Neutrons
Before you leave, take our quick quiz to enhance your learning!

