What is the atomic number of Scandium?
20
21
22
23

Scandium, a rare earth metal, is a fascinating element with a myriad of applications that extend from enhancing aerospace materials to revolutionizing modern technology. This lightweight metal, though not widely known, plays a pivotal role in strengthening alloys and giving them unique properties. Our comprehensive guide delves into the uses, benefits, and intriguing aspects of scandium, offering insightful examples to illuminate its significance. Whether you’re a science enthusiast or a professional in the field, understanding scandium’s potential can unlock new innovations and efficiencies in various industries. Join us as we explore the versatile and transformative world of scandium, shedding light on its applications, characteristics, and why it deserves more recognition in the scientific and technological spheres.
Scandium is a scarce, silvery metallic element with atomic number 21, primarily sourced from rare earth minerals. It’s not found freely in nature and is prized for its ability to enhance the properties of aluminum alloys, making them stronger and lighter. These alloys are crucial in the aerospace industry for manufacturing parts that offer improved performance and durability. Additionally, Scandium is utilized in high-intensity lighting and sports equipment, offering benefits like natural light mimicry and enhanced strength, respectively. Despite its versatility and utility across various industries, Scandium’s use is limited due to its rarity and the complexity of its extraction process.
Formula: Sc
Composition: Consists of a single scandium atom.
Bond Type: In its elemental form, scandium does not have bonds as it is a pure element. However, scandium can form covalent or ionic bonds when reacting with other elements.
Molecular Structure: As a pure element, scandium does not form a molecular structure in the same way as compounds like H?O. At room temperature, scandium is in a metallic state with a hexagonal close-packed crystalline structure.
Electron Sharing: In compounds, scandium typically shares electrons covalently or transfers electrons ionically, depending on the nature of the other element(s) it is bonding with.
Significance: Scandium is noted for its light weight and high melting point (1541°C or 2806°F), making it valuable in aerospace and military applications for materials that need to withstand extreme conditions. Its scarcity and difficulty in extraction contribute to its high value and limited use.
Role in Chemistry: Scandium’s most notable role is in the aerospace industry, where its alloys are used to create lightweight and strong materials. Additionally, scandium plays a role in the development of advanced ceramics and electronics, due to its ability to enhance the properties of materials such as aluminum. Its compounds are also used in the production of high-intensity lighting and in the realm of scientific research, particularly in organic chemistry as a catalyst in various reactions, demonstrating its versatility and importance in both industrial and research contexts.
The atomic structure of Scandium is a fascinating subject that lies at the intersection of chemistry and physics, offering insights into the behavior and characteristics of this intriguing element. Scandium, with the atomic symbol Sc and atomic number 21, is a transition metal known for its lightweight and high strength. Its atomic structure is key to understanding its unique properties and applications in various industries, including aerospace, electronics, and lighting.
Scandium’s electrons are arranged in shells around the nucleus, with each shell representing a different energy level. The distribution of electrons in Scandium’s shells is as follows:
This electron configuration highlights Scandium’s status as a transition metal, characterized by the filling of d orbitals.
Below is a table detailing the key physical properties of Scandium:
| Property | Value |
|---|---|
| Appearance | Silvery-white, metallic |
| Atomic Number | 21 |
| Atomic Weight | 44.955908 |
| Density | 2.985 g/cm³ at 20 °C |
| Melting Point | 1,541 °C (2,806 °F) |
| Boiling Point | 2,863 °C (5,185 °F) |
| State at 20 °C | Solid |
| Electrical Resistivity | 562 nanoohm-meters at 20 °C |
| Thermal Conductivity | 15.8 W/(m·K) at 300 K |
| Heat of Fusion | 14.1 kJ/mol |
| Heat of Vaporization | 332.7 kJ/mol |
| Specific Heat Capacity | 0.568 J/(g·K) |
| Electronegativity | 1.36 (Pauling scale) |
| Crystal Structure | Hexagonal close-packed (hcp) |
| Magnetic Ordering | Paramagnetic |
Scandium, with the atomic symbol Sc and atomic number 21, is a rare earth metal known for its application in aerospace and high-technology industries. This section explores the chemical properties of scandium, including its reactivity, common reactions, and compounds it forms.
Scandium is relatively soft and has a silver-white appearance that tarnishes in air, indicating its reactivity with oxygen. It reacts with water to form hydrogen gas and scandium hydroxide, showcasing its reactive nature.
Equation with Water:
Sc+3H2O?Sc(OH)3+3H2?
Scandium reacts with acids to produce hydrogen gas and the corresponding scandium salts. For instance, its reaction with hydrochloric acid yields scandium chloride.
Equation with Hydrochloric Acid:
Sc+?HCl?ScCl?+H??
At room temperature, scandium slowly oxidizes, forming scandium oxide. This reaction becomes more vigorous as the temperature increases.
Equation with Oxygen:
4Sc+3O??2Sc2O3?
Scandium forms alloys with a range of metals, including aluminum. These alloys are significantly stronger and have higher resistance to heat and corrosion. Scandium-aluminum alloys are particularly valued in aerospace and sports equipment manufacturing.
Scandium compounds, such as scandium triflate, act as catalysts in organic reactions, including the Friedel-Crafts alkylation. This underlines scandium’s importance in synthetic chemistry and materials science.
| Property | Value |
|---|---|
| Melting Point | 1541°C (2806°F) |
| Boiling Point | 2836°C (5137°F) |
| Heat of Fusion | 14.1 kJ/mol |
| Heat of Vaporization | 332.7 kJ/mol |
| Specific Heat Capacity | 25.52 J/(mol·K) |
| Thermal Conductivity | 15.8 W/(m·K) |
| Thermal Expansion | 10.2 µm/(m·K) (at 25°C) |
| Property | Value |
|---|---|
| Atomic Mass | 44.955908 u |
| Density | 2.985 g/cm³ (at 20°C) |
| Young’s Modulus | 74.4 GPa |
| Shear Modulus | 29.1 GPa |
| Bulk Modulus | 56.6 GPa |
| Poisson’s Ratio | 0.279 |
| Mohs Hardness | ? 6 |
| Brinell Hardness | 750 MPa |
| Property | Value |
|---|---|
| Electrical Resistivity | 562 n?·m (at 20°C) |
| Magnetic Ordering | Paramagnetic |
| Magnetic Susceptibility | +94.0·10?? cm³/mol (at 293 K) |
| Superconducting Point | < 1.22 K (not naturally occurring) |
| Property | Value |
|---|---|
| Atomic Number | 21 |
| Atomic Weight | 44.955908 u |
| Isotopes | Sc-45 (stable isotope) |
| Radioactive Isotopes | Sc-46, Sc-47, Sc-48, etc. |
| Half-Lives | Varies from milliseconds to days |
| Neutron Cross Section | 27.5 barns (for Sc-45) |
| Neutron Mass Absorption | 0.18 (for Sc-45) |
The preparation of scandium involves several sophisticated processes, given its rarity and the complexity of extracting it from its ores. Scandium is not found free in nature but in various minerals, with thortveitite (a scandium silicate) and uranium ores being the primary sources. The extraction and preparation process can be broken down into key steps to obtain pure scandium:
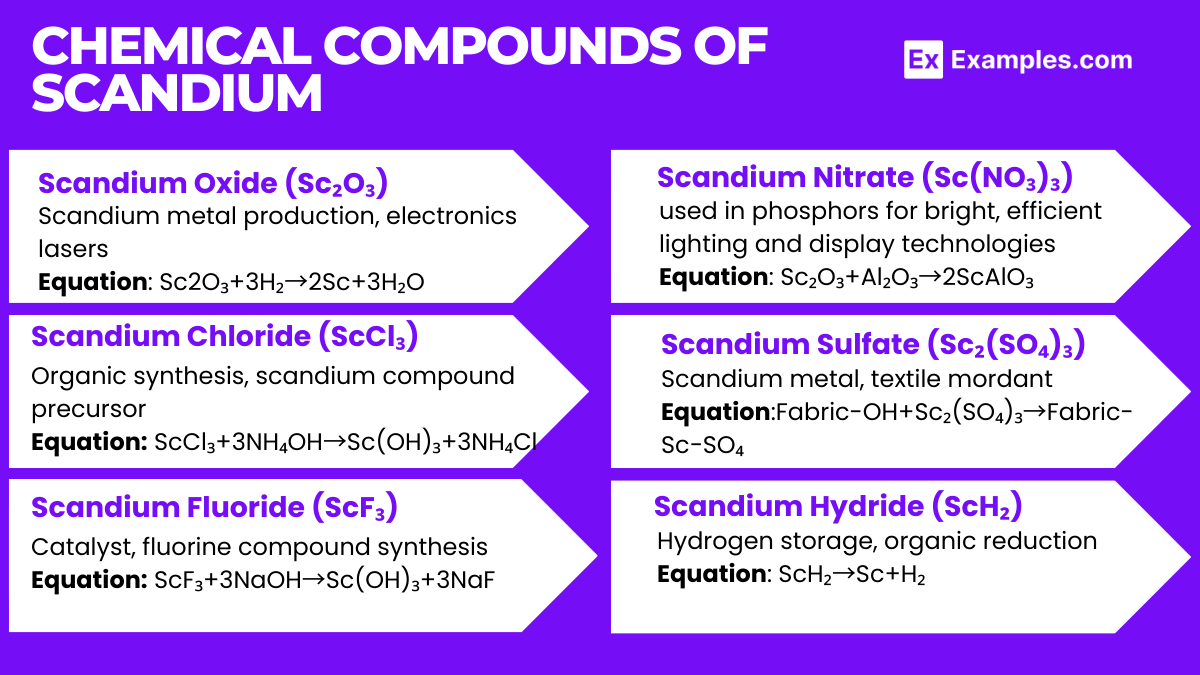
Scandium forms various compounds, each with unique properties and applications. Below are six scandium compounds, including their formulas and key details:
Scandium has one stable isotope and several radioactive isotopes. Below is a table summarizing the key isotopes of scandium, including their atomic mass, half-life, and mode of decay.
| Isotope | Atomic Mass | Half-Life | Mode of Decay |
|---|---|---|---|
| Sc-45 | 44.955912 | Stable | N/A |
| Sc-44 | 43.959402 | 3.97 hours | Electron capture to Ca-44 |
| Sc-46 | 45.955489 | 83.79 days | Beta decay to Ti-46 |
| Sc-47 | 46.952403 | 3.35 days | Beta decay to Ti-47 |
| Sc-48 | 47.952231 | 43.67 hours | Beta decay to Ti-48 |
Sc-45 is the only stable isotope and naturally occurring form of scandium. The radioactive isotopes, particularly Sc-46 and Sc-47, have applications in scientific research and medicine due to their radioactive properties.
Scandium is utilized across various fields, from aerospace to sports equipment, thanks to its unique properties. Below are the significant uses of scandium:
The production of Scandium is a multifaceted process, primarily sourced from thortveitite, a mineral, and as a byproduct of uranium and titanium processing. Despite its scattered presence in the Earth’s crust, Scandium’s extraction and refinement are challenging, leading to its classification as a rare earth element. The primary steps in Scandium production include:
The complexity and cost of these processes contribute to the relatively high price and limited production of Scandium worldwide.
Scandium’s unique properties enable its use in a diverse range of applications, significantly enhancing the performance of materials and products in various industries:
we explored the fascinating realm of scandium, delving into its unique physical and chemical properties, methods of preparation, and versatile applications. Through our comprehensive table, we’ve highlighted scandium’s significance in enhancing materials science and technology. Scandium, though rare and complex to extract, offers promising potential in various innovative fields, underscoring its growing importance in advancing modern technological solutions
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Scandium?
20
21
22
23
What is the chemical symbol for Scandium?
Sc
Sd
Sn
Sm
Scandium belongs to which group in the periodic table?
Group 1
Group 3
Group 5
Group 7
What is the most common oxidation state of Scandium in its compounds?
+1
+2
+3
+4
Which mineral is the primary source of Scandium?
Bauxite
Monazite
Ilmenite
Thortveitite
Scandium is primarily used in which industry?
Textile
Aerospace
Pharmaceutical
Agriculture
What is the melting point of Scandium?
660°C
1539°C
1812°C
2200°C
Scandium is classified as which type of element?
Alkali metal
Alkaline earth metal
Transition metal
Metalloid
What color does Scandium impart to flame in a flame test?
Red
Green
Yellow
Blue
Scandium is often alloyed with which metal to improve strength?
Aluminum
Copper
Iron
Zinc
Before you leave, take our quick quiz to enhance your learning!

