What is the atomic number of Tennessine?
115
116
117
118
Discover the fascinating world of Tennessine, the superheavy synthetic element that has captured the attention of scientists and chemistry enthusiasts alike. In this comprehensive guide, we dive into the mysteries and marvels of Tennessine, exploring its unique properties, synthesis, and potential applications. With expert insights and engaging examples, we’ll unravel the significance of this remarkable element in the periodic table. Whether you’re a seasoned chemist or simply curious about the latest advancements in material science, our exploration of Tennessine promises to enlighten and inspire. Join us as we delve into the atomic intricacies and groundbreaking research surrounding one of the newest members of the periodic family, ensuring a captivating journey through the world of modern chemistry.
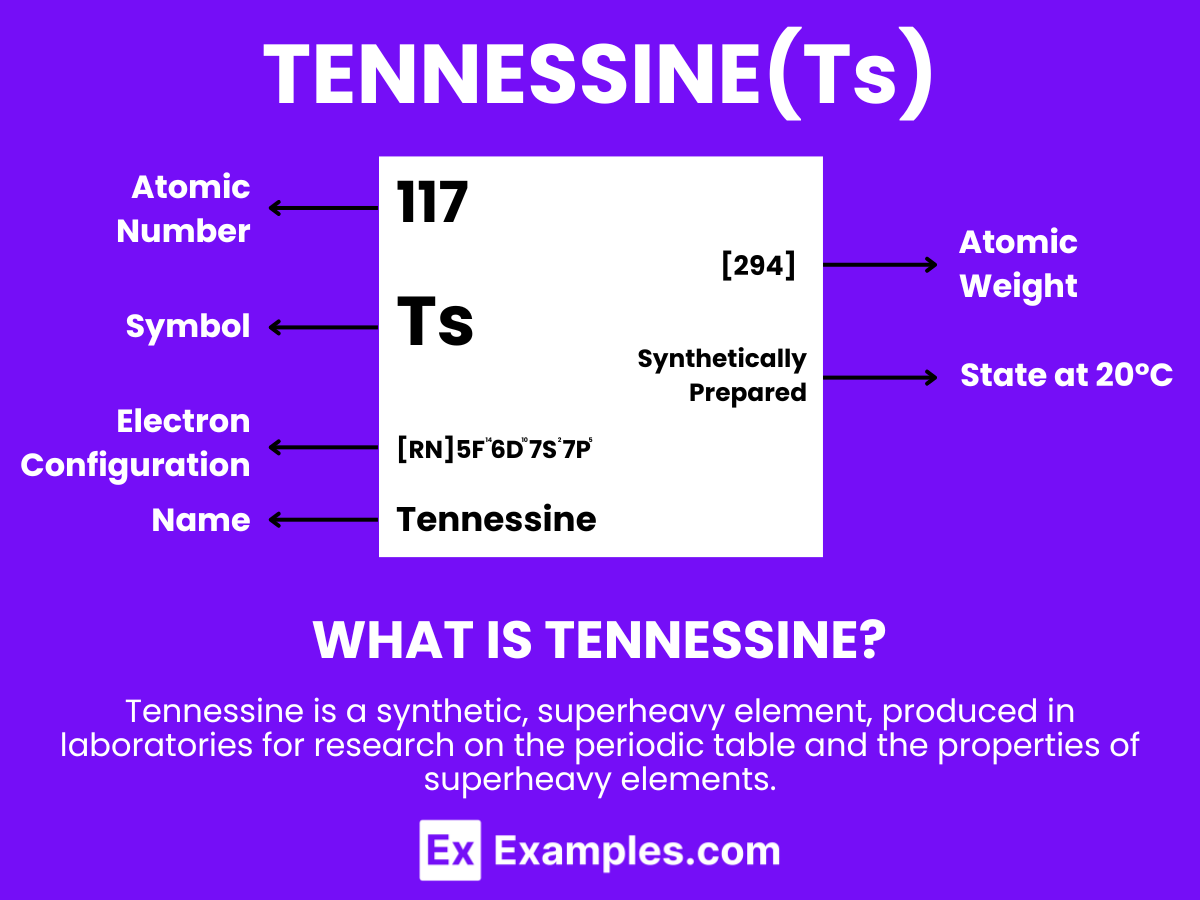
Tennessine is a superheavy, synthetic element with the chemical symbol Ts and atomic number 117. It is known for being produced in particle accelerators through the fusion of atomic nuclei. Tennessine does not occur in nature and has a very short lifespan before it decays, which presents challenges for its study. The element’s discovery is crucial for nuclear physics research, especially in probing the properties and behaviors of superheavy elements in the periodic table. Because of its significant instability and radioactivity, tennessine has no practical applications beyond scientific inquiry, where it plays a role in investigating the conjectural “island of stability” and the boundaries of the periodic table.
Tennessine, a synthetic element positioned significantly apart from lighter, more commonly encountered elements such as hydrogen or gallium, is a superheavy element that holds a distinctive place in nuclear chemistry due to its location in the periodic table and its classification as a post-actinide.
Atomic Level: Each atom of Tennessine (Ts) is characterized by having 117 protons in its nucleus, defining its atomic number as 117. The theoretical electron configuration of Tennessine is [Rn]5f¹⁴ 6d¹⁰ 7s² 7p⁵, indicating it has a full 5f and 6d orbital, with five electrons in its 7p orbital, setting the stage for chemical interactions. However, relativistic effects are expected to significantly influence its actual electron configuration, potentially altering its chemical properties.
Molecular Formation: Unlike simpler elements that can form diatomic molecules (such as H₂), Tennessine does not naturally form molecules or exhibit a stable molecular structure due to its extremely short half-life and high instability. The element exists for only milliseconds before decaying into lighter elements, making the study of its bonding characteristics and molecular formation largely theoretical. In the hypothetical scenario where Tennessine atoms could persist long enough to interact chemically, their behavior would likely be influenced by their electron configuration, but this remains speculative.
The stability and phase of Tennessine under various temperatures and pressures are subjects of theoretical speculation, as its brief existence precludes the observation of solid, liquid, or gaseous states under normal conditions. The term “Tennessine Gas” does not apply in the same way it might for compounds like uranium hexafluoride (UF₆) in the context of uranium.
| Property | Description |
|---|---|
| Appearance | Not observed directly; assumed to have no stable or long-lasting physical form due to its extreme radioactivity |
| Atomic Number | 117 |
| Density (at 20°C) | d 7.1-7.3 g/cm³ |
| Melting Point | Predicted range: Not specifically estimated, but expected to be high (theoretical) |
| Boiling Point | Predicted to be high, specific values not estimated (theoretical) |
| State at Room Temperature | Expected to be solid (based on theoretical calculations) |
| Electron Configuration | [Rn] 5f¹⁴ 6d¹⁰ ₇s² ₇p⁵ |
| Common Oxidation States | +1, +3, +5 |
Tennessine, with the atomic number 117, is a synthetic element situated in group 17 of the periodic table.
The exploration of tennessine’s chemical properties remains largely theoretical, awaiting advancements in experimental techniques and the production of more stable isotopes for in-depth study.
| Property | Value with Unit |
|---|---|
| Atomic Number | 117 |
| Atomic Mass | Most stable isotope: Tennessine-294 (294 u) |
| Isotopes | ^294Ts (most stable), among others |
| Half-Life (for ^294Ts) | ~20 milliseconds (estimated) |
| Nuclear Spin | Not precisely determined due to short half-lives |
| Neutron Cross Section | Not determined (extremely short-lived isotopes make measurement challenging) |
Tennessine is a superheavy, synthetic element that does not occur naturally and can only be synthesized in a laboratory setting. The preparation of tennessine involves highly specialized equipment, including advanced nuclear reactors and ion accelerators. Here is an outline of the general process used to create tennessine:
Selection of Target and Projectile:
Acceleration:
Collision and Fusion:
Nucleus Cooling and Decay:
Detection and Identification:
Isolation of Isotopes:
A theoretical compound suggesting the interaction between tennessine and oxygen to form an oxide.
Equation: 2Ts + O₂ → TsO₂
Predicts the formation of a fluoride compound when tennessine reacts with fluorine.
Equation: 2Ts + F₂ → TsF₂
Suggests the possibility of tennessine combining with chlorine to form a chloride compound.
Equation: 2Ts + Cl₂ → TsCl₂
Indicates the theoretical reaction between tennessine and bromine to produce a bromide.
Equation: 2Ts + Br₂ → TsBr₂
Inference about tennessine’s ability to react with iodine to form an iodide compound.
Equation: 2Ts + I₂ → TsI₂
Speculates on the reaction between tennessine and hydrogen to create a hydride.
Equation: 2Ts + H₂ → TsH₂
Tennessine is a synthetic element with no stable isotopes. Its isotopes have been created in laboratory settings through nuclear reactions, showcasing distinct decay characteristics.
| Isotope | Half-Life | Decay Mode |
|---|---|---|
| Ts-293 | Less than 20 ms | Alpha decay to Mc-289 |
| Ts-294 | ~51 milliseconds | Alpha decay to Mc-290 |
| Ts-295 | Predicted, not observed | Predicted alpha decay |
| Ts-296 | Predicted, not observed | Predicted alpha decay |
| Ts-297 | Predicted, not observed | Predicted alpha decay to Mc-293 |
| Ts-298 | Predicted, not observed | Predicted alpha decay |
| Ts-299 | Predicted, not observed | Predicted alpha decay |
Tennessine is a synthetic, superheavy element with the atomic number 117. Due to its extremely short half-life and the fact that it can only be produced in minute quantities. Below are the potential uses and areas of interest related to tennessine:
Nuclear Physics Research:
Chemical Element Research:
Investigation of Relativistic Effects:
Astrophysical Research:
Development of New Materials and Technologies:
Tennessine, with the symbol Ts and atomic number 117, is a synthetic element that is produced in minuscule quantities through nuclear reactions involving heavy ions. Here is an outline of how tennessine is produced:
Tennessine is a synthetic element with the atomic number 117, identified by its symbol, Ts. Due to its extremely short half-life and the challenges associated with its production in very small quantities:
Tennessine is a fascinating element that pushes the boundaries of our understanding of the periodic table. As a member of the halogen group, it challenges traditional notions with its unique and theoretical properties. Although largely unexplored due to its short half-life, Tennessine’s synthesis marks a significant achievement in nuclear chemistry, offering a glimpse into the behavior of superheavy elements and the potential for future discoveries in this intriguing area of science.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Tennessine?
115
116
117
118
In which group of the periodic table is Tennessine found?
Group 15
Group 16
Group 17
Group 18
What is the symbol for Tennessine on the periodic table?
Ts
Te
Tn
Tm
Which element is Tennessine named after?
Tennessee
Tenure
Tenness
Tennen
What type of element is Tennessine?
Alkali metal
Alkaline earth metal
Transition metal
Halogen
What is the most stable isotope of Tennessine?
Ts-294
Ts-293
Ts-292
Ts-295
What is the predicted oxidation state of Tennessine?
-1
0
+1
+3
How was Tennessine synthesized?
Nuclear fusion
Chemical reaction
Electrolysis
Thermal decomposition
Tennessine is expected to be similar to which other element?
Nitrogen
Oxygen
Iodine
Xenon
What is the half-life of the most stable isotope of Tennessine?
20 seconds
40 seconds
80 seconds
120 seconds
Before you leave, take our quick quiz to enhance your learning!

