What is the atomic number of terbium?
65
66
67
68

Dive into the fascinating world of Terbium, a rare earth element that’s silently revolutionizing technology and green energy solutions. This complete guide sheds light on Terbium’s definition, intriguing uses, and the compelling compounds it forms. From enhancing the vibrancy of your smartphone’s display to playing a critical role in cutting-edge medical diagnostics, Terbium’s versatility is unmatched. Through practical examples, discover how this luminous element not only powers our daily gadgets but also paves the way for sustainable innovations. Explore the essence and impact of Terbium in today’s tech-driven era.
Terbium is a chemical element with the symbol Tb and atomic number 65. It is a silvery-white, rare earth metal that belongs to the lanthanide series of the periodic table. Terbium is not found in nature as a free element but is extracted from various minerals, such as cerite, gadolinite, and monazite, which contain small amounts of multiple rare earth elements.Terbium has unique physical and chemical properties that make it valuable in various applications. It has excellent magnetic and fluorescent properties, which are exploited in the development of electronics, such as in the production of green phosphors used in color TV tubes and LED lights, and in solid-state devices like sensors and actuators. Additionally, Terbium alloys are used in the production of electronic devices because of their ability to crystallize in a magnetostrictive form, which changes shape under the influence of a magnetic field.
Formula: Tb
The atomic structure of Terbium (Tb), a rare earth element with atomic number 65, plays a crucial role in defining its chemical and physical properties. Here’s a detailed look into its atomic structure:
| Property | Value |
|---|---|
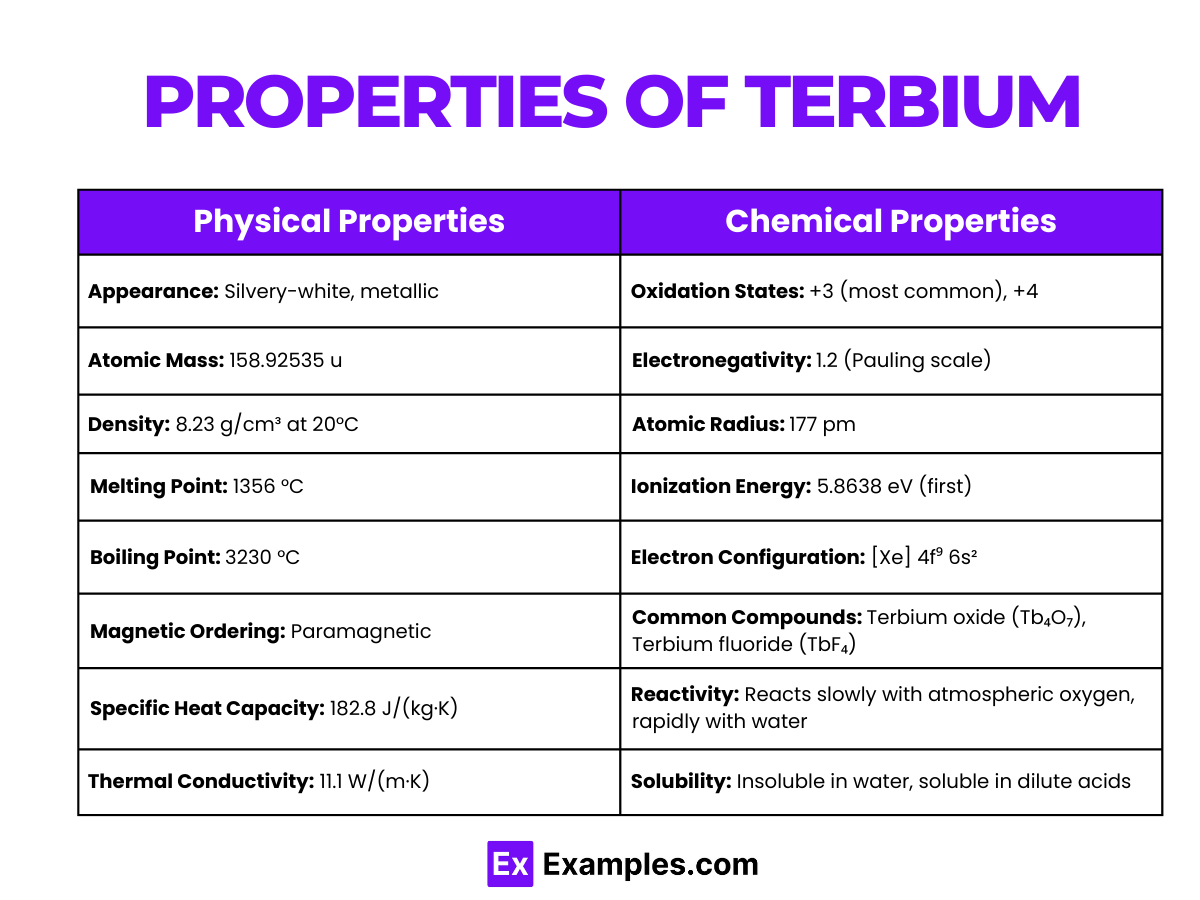
| Appearance | Silvery-white, metallic |
| Atomic Mass | 158.92535 u |
| Density | 8.23 g/cm³ at 20°C |
| Melting Point | 1356 °C |
| Boiling Point | 3230 °C |
| Magnetic Ordering | Paramagnetic |
| Specific Heat Capacity | 182.8 J/(kg·K) |
| Thermal Conductivity | 11.1 W/(m·K) |
| Electrical Resistivity | ~1.150 µΩ·m (at 20 °C) |
Terbium, a member of the lanthanide series, showcases a set of chemical properties that make it notable for its use in various applications. Here’s a detailed look at its chemical properties, accompanied by relevant chemical equations:
| Property | Value |
|---|---|
| Melting Point | 1356 °C |
| Boiling Point | 3230 °C |
| Specific Heat Capacity | 182.8 J/(kg·K) |
| Thermal Conductivity | 11.1 W/(m·K) |
| Thermal Expansion | 10.3 µm/(m·K) (at 25 °C) |
| Heat of Fusion | 10.15 kJ/mol |
| Heat of Vaporization | 391 kJ/mol |
| Entropy of Fusion | 17.0 J/(mol·K) (at melting point) |
| Property | Value |
|---|---|
| Density | 8.23 g/cm³ (at 20 °C) |
| Mohs Hardness | Approximately 2.5 |
| Young’s Modulus | 55.7 GPa |
| Shear Modulus | 22.1 GPa |
| Bulk Modulus | 38.7 GPa |
| Poisson’s Ratio | 0.261 |
| Vickers Hardness | 677 HV |
| Brinell Hardness | 600 – 1050 HB |
| Property | Value |
|---|---|
| Magnetic Ordering | Paramagnetic (above 219 K) |
| Curie Temperature | 219 K |
| Magnetic Susceptibility | High in +3 oxidation state |
| Electrical Resistivity | ~1.150 µΩ·m (at 20 °C) |
| Superconducting Point | Not a superconductor |
| Property | Value |
|---|---|
| Natural Isotopes | ¹⁵⁹Tb (100% natural abundance) |
| Radioactive Isotopes | ¹⁵⁸Tb, ¹⁶⁰Tb (among others) |
| Neutron Cross Section | 23.3 barns (for ¹⁵⁹Tb) |
| Neutron Mass Absorption | 0.0046 |
| Isotopic Abundance | ¹⁵⁹Tb: 100% |
| Isotope | Mass Number | Half-Life | Decay Mode | Application/Significance |
|---|---|---|---|---|
| ¹⁵⁰Tb | 150 | 3.48 hours | Beta decay | Research |
| ¹⁵¹Tb | 151 | 17.609 hours | Beta decay | Medical research, cancer treatment |
| ¹⁵²Tb | 152 | 17.5 hours | Beta decay | Medical imaging and therapy |
| ¹⁵³Tb | 153 | 2.34 days | Beta decay | Research, potential therapeutic uses |
| ¹⁵⁴Tb | 154 | 21.5 hours | Beta decay | Research |
| ¹⁵⁹Tb | 159 | Stable | – | Most common and naturally occurring |
| ¹⁶⁰Tb | 160 | 72.3 days | Beta decay | Research |
Terbium has a variety of isotopes, both stable and radioactive. Among these, ¹⁵⁹Tb is the only naturally occurring and stable isotope, which makes it the most significant for practical applications. The radioactive isotopes of terbium, such as ¹⁵¹Tb and ¹⁵²Tb, have potential and actual uses in medical research, particularly in cancer treatment and diagnostic imaging due to their radioactive decay properties.
The production of terbium is a complex process, largely because terbium is found in small quantities within mixed rare earth minerals such as monazite and bastnäsite. These steps outline the general production process:
Terbium, a lanthanide series element, is highly valued for its unique physical and chemical properties, leading to diverse applications across various industries:
Terbium’s remarkable properties and versatile applications make it a pivotal element in advancing modern technology. From enhancing display technologies with vibrant colors to improving the efficiency of LED lighting and contributing to medical imaging, terbium’s role is irreplaceable. This table of terbium not only highlights its diverse uses but also underscores its significance in shaping a sustainable and technologically advanced future.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of terbium?
65
66
67
68
Terbium belongs to which group of elements in the periodic table?
Alkali metals
Alkaline earth metals
Transition metals
Lanthanides
What is the chemical symbol for terbium?
Tb
Tr
Tm
Tc
Which of the following is a common use of terbium?
Fuel
Lighting phosphors
Fertilizer
Lubricant
Terbium was discovered in which country?
France
Germany
Sweden
England
Terbium is most commonly found in which type of ore?
Bauxite
Hematite
Cassiterite
Monazite
What is the melting point of terbium?
1021°C
1356°C
1530°C
2470°C
Which of the following is a property of terbium?
Highly reactive
Magnetic
Brittle
Poor conductor
Terbium is used in which type of medical imaging?
MRI
X-ray
Ultrasound
PET scan
Terbium ions can exhibit which color in fluorescence?
Red
Green
Blue
Yellow
Before you leave, take our quick quiz to enhance your learning!

