Thulium belongs to which group of elements?
Alkali metals
Alkaline earth metals
Lanthanides
Actinides
On a fascinating journey through the realm of Thulium, a lesser-known yet incredibly significant element in the periodic table. This complete guide offers a deep dive into the definition, meaning, and myriad uses of Thulium, alongside an exploration of its compounds. With examples to illuminate its role in science and technology, this introduction aims to enrich your understanding of Thulium, highlighting its unique properties and the innovative ways it enriches our world. Perfect for enthusiasts and experts alike, this guide will enhance your knowledge and appreciation of this rare, silvery metal.
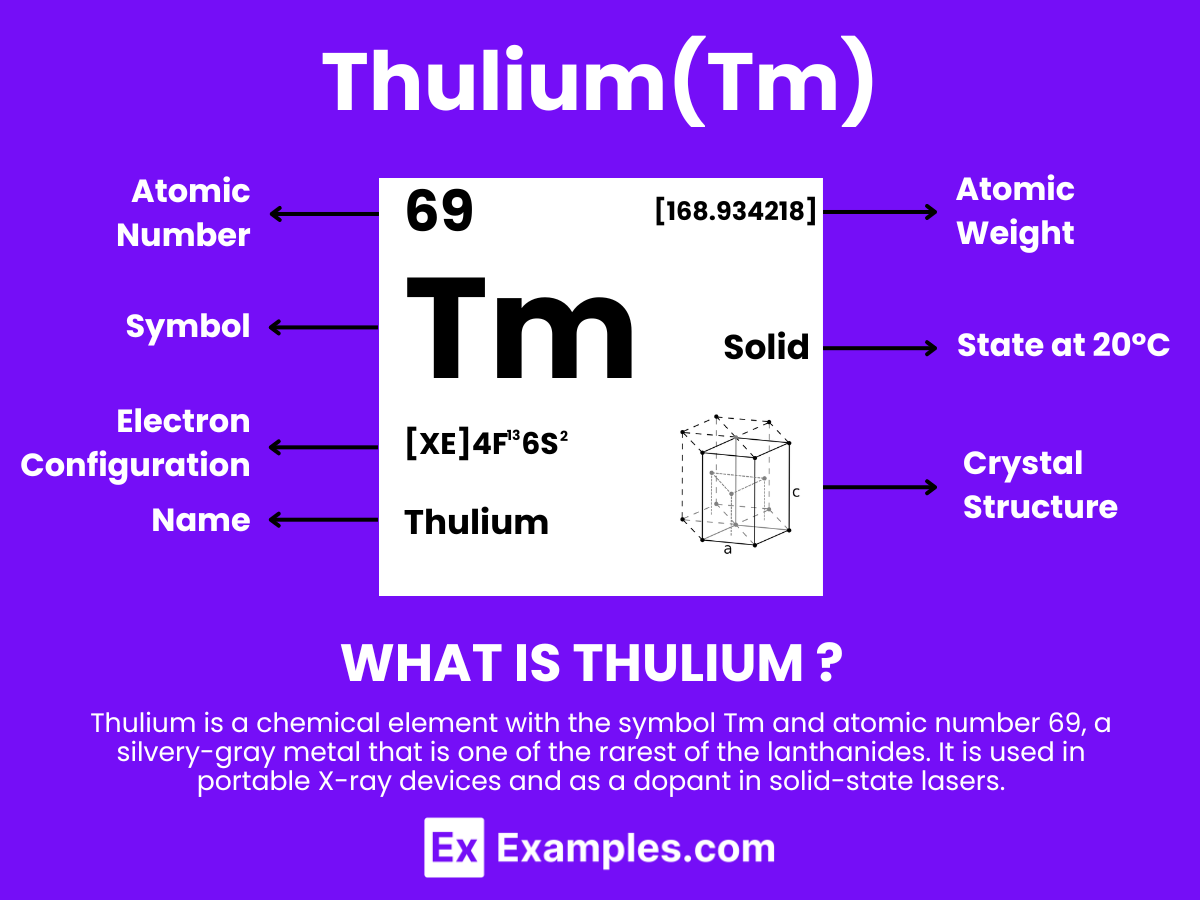
Thulium is a chemical element with the symbol Tm and atomic number 69. It is a member of the lanthanide series in the periodic table, a group of elements known as rare earth metals. Thulium is the thirteenth and third-last element in the lanthanide series. Despite its classification as a rare earth element, thulium is fairly abundant in the Earth’s crust, though it is not found in free form in nature. It is usually extracted from minerals such as monazite and xenotime, where it occurs in small amounts.
Thulium has a bright, silvery-gray appearance and is relatively soft and malleable. It can be cut with a knife when pure. The element has a melting point of 1545 degrees Celsius and a boiling point of 1950 degrees Celsius. Among the lanthanides, thulium is noted for its low level of radioactivity; its most stable isotope, Thulium-169, is not radioactive and does not pose a significant risk in terms of radioactivity.
Thulium, in contrast to hydrogen, is a metallic element with established characteristics that include a stable solid form at room temperature and unique properties stemming from its status as a rare earth metal. The behavior of thulium at the atomic and molecular levels significantly diverges from that of hydrogen, given its position as a lanthanide in the periodic table and its metallic characteristics.
Atomic Level: Each thulium atom (Tm) contains 69 protons in its nucleus and is expected to have 69 electrons orbiting around it. The electron configuration of thulium is [Xe] 4f¹³ 6s², indicating a relatively complex electron configuration with a potential for various oxidation states, though +3 is the most common and stable. This indicates a level of chemical reactivity typical of lanthanides and the possibility for forming compounds, particularly with elements that can achieve stable complementary oxidation states.
Molecular Formation: Unlike hydrogen, which forms simple molecules like H₂ through covalent bonding, thulium would not form molecules in a similar manner due to its metallic nature. In bulk form, thulium exhibits a metallic lattice structure typical of metals, involving a hexagonal close-packed (hcp) arrangement. This structure involves metallic bonding, where electrons are delocalized over many thulium atoms, differing fundamentally from the discrete electron sharing seen in hydrogen’s covalent bonds. Thulium’s solid form is stable and can be observed directly, unlike the hypothetical and highly unstable forms of more radioactive elements like bohrium.
| Property | Value |
|---|---|
| Atomic Number | 69 |
| Atomic Mass | 168.93422 g/mol |
| Density | 9.32 g/cm³ at 20°C |
| Melting Point | 1545 °C |
| Boiling Point | 1950 °C |
| State at Room Temperature | Solid |
| Color | Silvery gray |
| Thermal Conductivity | 16.9 W/(m·K) |
| Electrical Resistivity | 676 nΩ·m at 20°C |
Thulium, like other lanthanides, exhibits a +3 oxidation state in most of its compounds, indicative of its chemical stability and reactivity. Thulium compounds are generally formed by reacting the metal with various non-metals or by dissolving thulium in acids. Here are some examples illustrating thulium’s chemical properties:
This oxide is a common starting material for producing other thulium compounds.
This demonstrates its reactivity and the ease of forming salts.
| Property | Value |
|---|---|
| Standard Atomic Weight | 168.93422 g/mol |
| Heat of Fusion | 16.84 kJ/mol |
| Heat of Vaporization | 251 kJ/mol |
| Specific Heat Capacity | 27.03 J/(mol·K) |
| Thermal Expansion | 13.3 µm/(m·K) at 25°C |
| Property | Value |
|---|---|
| Crystal Structure | Hexagonal Close-Packed (hcp) |
| Hardness | Soft (can be cut with a knife) |
| Modulus of Elasticity | 74.0 GPa |
| Poisson’s Ratio | 0.231 (Estimated) |
| Ductility | High, malleable and ductile |
| Corrosion Resistance | Good, especially in dry air |
| Property | Description |
|---|---|
| Electrical Resistivity | High; about 676 nΩ·m at room temperature |
| Magnetic Ordering | Paramagnetic |
| Thermal Conductivity | 16.9 W/(m·K) at room temperature |
| Magnetic Susceptibility | Positive; paramagnetic at room temperature |
| Superconducting Point | Below 1.5 K, thulium becomes superconducting |
| Optical Properties | Absorbs in the visible and near-infrared; used in lasers |
| Property | Description |
|---|---|
| Natural Isotopes | Thulium-169 (100% abundance) |
| Radioactive Isotopes | Tm-170, Tm-171, among others; all synthetic |
| Nuclear Spin (Tm-169) | 1/2 |
| Neutron Cross Section | 100 barns for thermal neutrons |
| Isotope Mass | 168.93422 u for Tm-169 (stable isotope) |
| Half-Life of Most Stable Radioisotope (Tm-171) | 1.92 years |
| Decay Modes | Beta decay for radioactive isotopes |
| Nuclear Quadrupole Moment | Thulium nuclei possess a nuclear quadrupole moment, useful in nuclear magnetic resonance (NMR) |
The preparation of thulium, a rare earth element, involves several steps to extract and purify it from its ores. The primary sources of thulium are monazite and xenotime sands, which contain a variety of rare earth elements. The extraction process typically includes:
| Isotope | Mass Number | Half-life | Primary Decay Mode |
|---|---|---|---|
| Tm-167 | 167 | Stable | – |
| Tm-168 | 168 | 93 days | Electron Capture |
| Tm-169 | 169 | Stable | – |
| Tm-170 | 170 | 128.6 days | Beta Decay |
| Tm-171 | 171 | 1.92 years | Beta Decay |
| Tm-172 | 172 | 63.6 hours | Beta Decay |
| Tm-173 | 173 | 8.24 years | Beta Decay |
| Tm-174 | 174 | 142 days | Beta Decay |
Thulium is one of the least abundant rare earth metals and is primarily extracted from monazite and xenotime ores, which contain small amounts of all the rare earth elements. The production process involves several key steps:
Thulium has a variety of specialized applications, owing to its unique properties:
Article delves into thulium, outlining its preparation, chemical compounds, and their respective properties and equations. Through detailed exploration, we’ve unveiled thulium’s significant role in technology and science, highlighting its versatility and utility in various fields. Our comprehensive guide illustrates thulium’s contribution to advancements in materials science, showcasing its unique characteristics and the potential for future applications.
Text prompt
Add Tone
Production of Thulium
Uses of Thulium
Thulium belongs to which group of elements?
Alkali metals
Alkaline earth metals
Lanthanides
Actinides
What is the symbol for thulium?
Th
Tm
Tl
Te
What is the standard state of thulium at room temperature?
Gas
Liquid
Solid
Plasma
Thulium is primarily used in which type of applications?
Fuel production
Medical imaging
Construction
Agriculture
Thulium is most commonly extracted from which type of mineral?
Bauxite
Monazite
Cassiterite
Hematite
What color does thulium exhibit in its pure form?
Silvery-gray
Gold
Black
White
What is the melting point of thulium?
1350°C
1545°C
1818°C
2500°C
Thulium is considered to be:
Highly reactive
Moderately reactive
Low reactive
Non-reactive
What is the primary health hazard associated with thulium?
Toxicity
Radioactivity
Corrosiveness
Carcinogenicity
Thulium is used as a source of radiation in which device?
MRI machines
X-ray machines
Gamma cameras
Portable X-ray devices
Before you leave, take our quick quiz to enhance your learning!

