What is the atomic number of Tungsten?
72
74
76
78
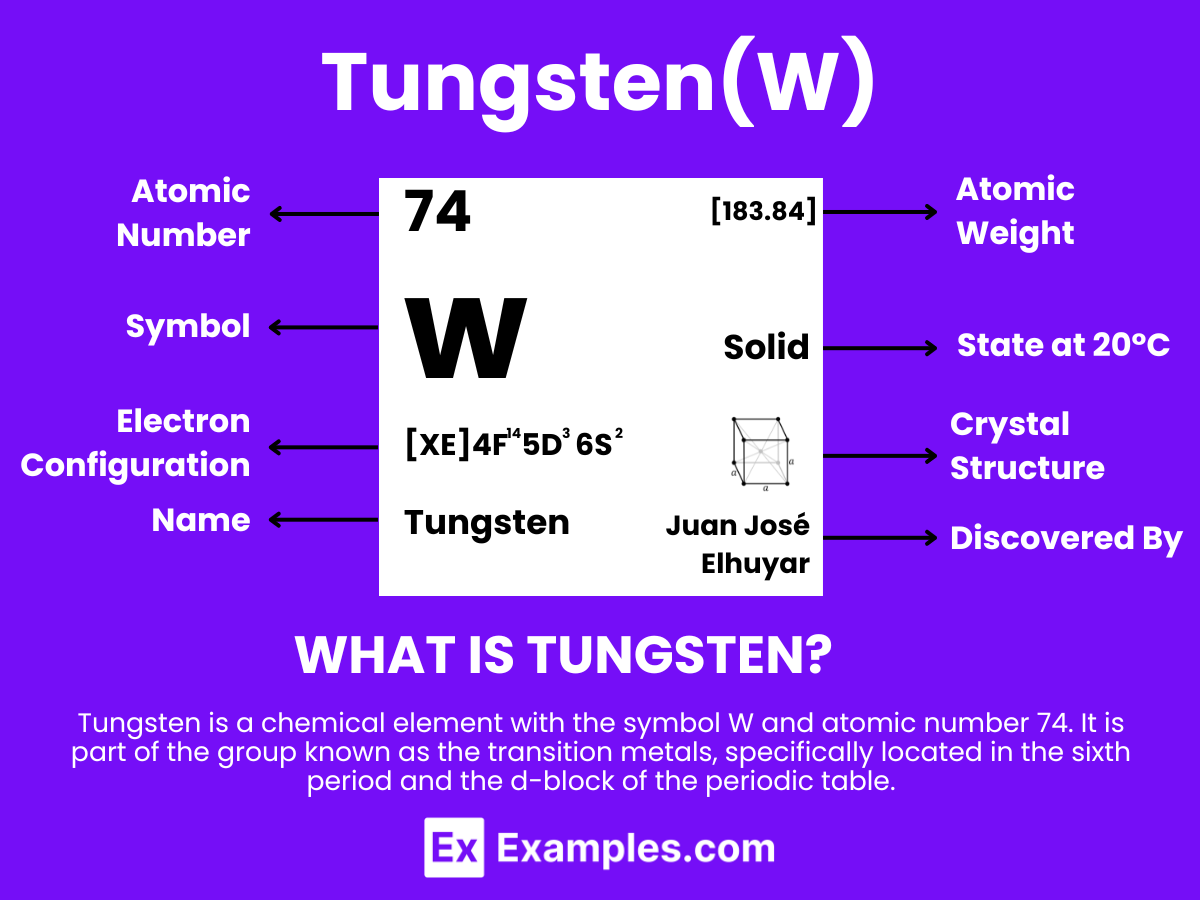
Dive into the remarkable world of Tungsten, an element renowned for its exceptional strength and high melting point. This complete guide illuminates Tungsten’s role in modern technology and industry, from its critical use in light bulb filaments to its significance in aerospace design. With a detailed exploration of its chemical properties and a rich array of applications, this introduction to Tungsten will provide a comprehensive understanding of why this metal is so integral to advancements in engineering and manufacturing. Embrace the journey through the atomic structure and versatile uses of Tungsten, and discover the innovative compounds that highlight its unique place in the periodic table.
Tungsten is a metallic element with the chemical symbol W and atomic number 74. It is extracted from minerals such as wolframite and scheelite. Tungsten has the highest melting point of all the elements discovered, standing out for its exceptional strength and high density, which render it indispensable in various industrial and technological applications. The discovery of tungsten was significant in the field of chemistry for its contribution to the understanding of transition metals in the periodic table. Its remarkable ability to withstand extreme temperatures makes it crucial in the manufacturing of products requiring high thermal stability.
Exploring the atomic structure of Tungsten uncovers the essence of its remarkable durability and broad applicational scope. With 74 protons in its nucleus, Tungsten’s atomic characteristics illuminate its indispensability in high-tech and industrial applications.
The stability and behavior of Tungsten under different temperatures and pressures are well-documented, showcasing its solid state under standard conditions. Its exceptional melting point and durability at elevated temperatures highlight its utility in challenging environments, such as in aerospace, military, and industrial technologies, cementing Tungsten’s role in advancing contemporary science and industrial capabilities.
| Property | Value |
|---|---|
| Atomic Number | 74 |
| Atomic Weight | 183.84 |
| Density | 19.25 g/cm³ |
| Melting Point | 3422 °C |
| Boiling Point | 5555 °C |
| Mohs Hardness | 7.5 |
| Tensile Strength | 1510 MPa |
| Thermal Conductivity | 173 W/(m·K) |
| Electrical Resistivity | 5.6 x 10^-⁸ Ω·m |
Tungsten’s chemical properties are as remarkable as its physical ones. It is characterized by its exceptional resistance to corrosion and high temperatures.
| Property | Value |
|---|---|
| Melting Point | 3422 |
| Boiling Point | 5555 |
| Heat of Fusion | 35.3 |
| Heat of Vaporization | 806 |
| Specific Heat Capacity | 0.134 |
| Thermal Conductivity | 173 |
| Property | Value |
|---|---|
| Density | 19.25 |
| Mohs Hardness | 7.5 |
| Tensile Strength | 1510 |
| Young’s Modulus | 411 |
| Poisson’s Ratio | 0.28 |
| Vickers Hardness | 3430 |
| Property | Value |
|---|---|
| Electrical Resistivity | 5.6 x 10^-⁸ |
| Thermal Conductivity | 173 |
| Magnetic Susceptibility | -0.00012 |
| Property | Value |
|---|---|
| Atomic Number | 74 |
| Atomic Mass | 183.84 |
| Cross Section for Thermal Neutrons | 19.2 |
| Isotopes | 180, 182, 183, 184, 186 |
Tungsten carbide is renowned for its extreme hardness and high melting point.
Equation: W+C→WC
Tungsten forms several oxides, including tungsten(IV) oxide (WO₂) and tungsten(VI) oxide (WO₃).
Equation: WO₃+10NH₃+11H₂O
Tungsten forms a series of volatile halides, including hexachloride (WCl₆) and hexafluoride (WF₆).
Equation: 6W+3Cl₂→WCl₆
Tungsten disulfide is a dichalcogenide compound with a layered structure similar to graphite.
Equation: 2W+2S→WS₂
Tungsten hexacarbonyl is an organometallic compound with a metal center surrounded by six carbonyl (CO) ligands.
Equation: W(CO)₆+6Cl₂
Tungsten trioxide hydrate is a hydrated form of tungsten(VI) oxide.
Equation: WO₃⋅H₂O+10NH₄++10OH
| Isotope | Half-life | Mode of Decay |
|---|---|---|
| W-180 | Stable | Stable (non-radioactive) |
| W-182 | Stable | Stable (non-radioactive) |
| W-183 | Stable | Stable (non-radioactive) |
| W-184 | Stable | Stable (non-radioactive) |
| W-186 | Stable | Stable (non-radioactive) |
This comprehensive overview presents Tungsten as a highly valuable element, essential to various high-tech industries due to its unparalleled thermal stability and high density. Tungsten’s capacity to endure extreme conditions and resist wear makes it indispensable in nuclear reactors, aerospace, electronics, and the medical field, furthering our scientific knowledge and technological progress.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Tungsten?
72
74
76
78
What is the chemical symbol for Tungsten?
Tu
Tg
W
Tn
Tungsten has the highest melting point of all metals. What is its melting point?
3,422°C
2,870°C
4,023°C
3,150°C
Which of the following is a common use for Tungsten?
Electrical wiring
Filaments in light bulbs
Jewelry
Batteries
Tungsten is also known for its use in which high-strength applications?
Aircraft components
Construction materials
Tool steels and cutting tools
Plastic manufacturing
What is the density of Tungsten?
10.5 g/cm³
15.6 g/cm³
19.3 g/cm³
21.4 g/cm³
Which of the following is a property of Tungsten?
Low thermal conductivity
High reactivity with acids
Low density
High melting point
Tungsten can be alloyed with which metal to make superalloys used in jet engines?
Aluminum
Copper
Nickel
Zinc
What is the primary industrial method used to extract Tungsten from its ores?
Electrolysis
Smelting
Leaching
Roasting and reduction
Tungsten is used in radiation shielding due to its
Low density
High atomic number
High reflectivity
High malleability
Before you leave, take our quick quiz to enhance your learning!

