What is the atomic number of Ununennium?
118
119
120
121
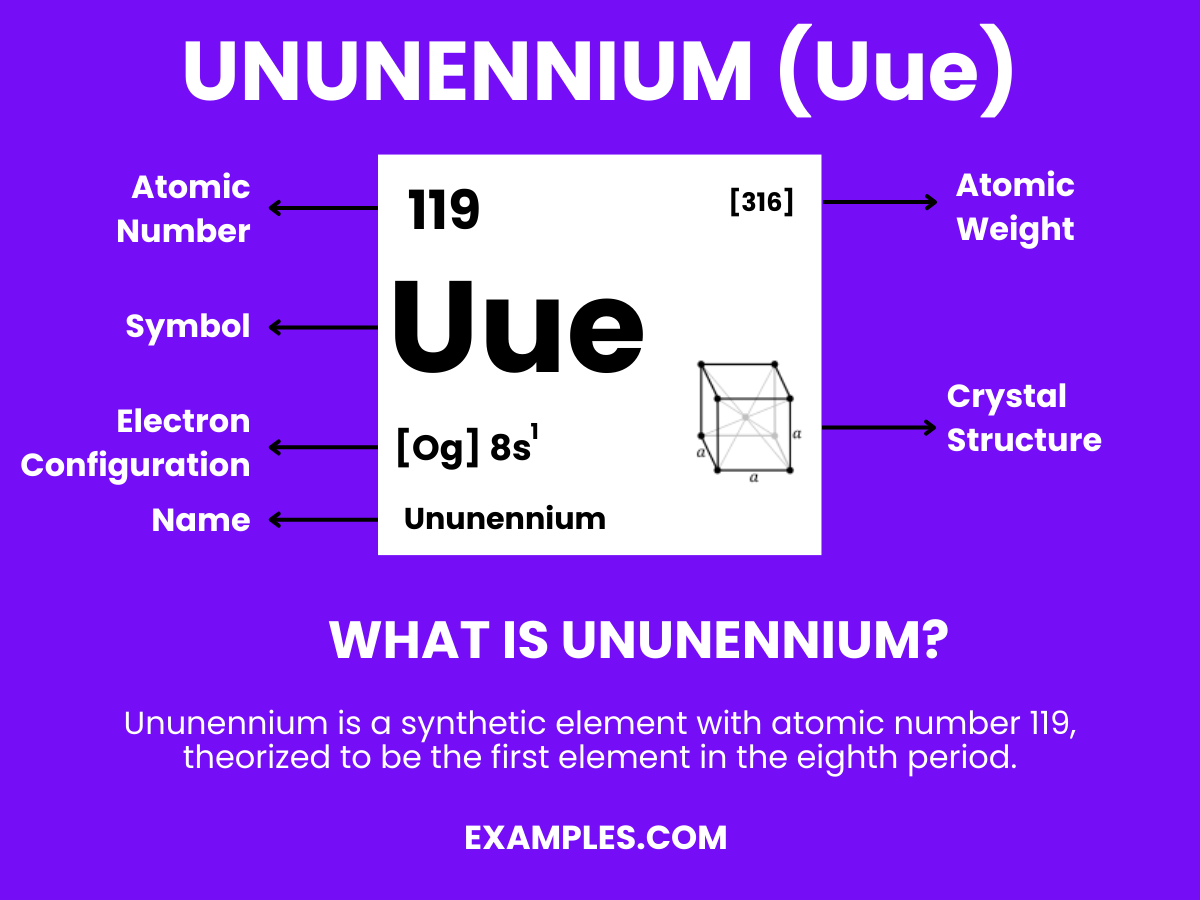
In the fascinating world of chemistry, there are elements that capture our imagination with their unique characteristics, and Ununennium is one such element. It stands out in the periodic table not because it’s found in nature, but because it is entirely man-made, a testament to human ingenuity in the field of science. Ununennium is part of an exclusive club known as superheavy elements, boasting an atomic number that places it in the realm of the unknown and uncharted. This element’s existence is fleeting, lasting only moments before it decays, yet its discovery and study offer invaluable insights into the boundaries of chemical science and the potential for new, yet-to-be-discovered elements. Ununennium’s presence enriches our understanding of chemistry, pushing the limits of what we know about the building blocks of the universe.
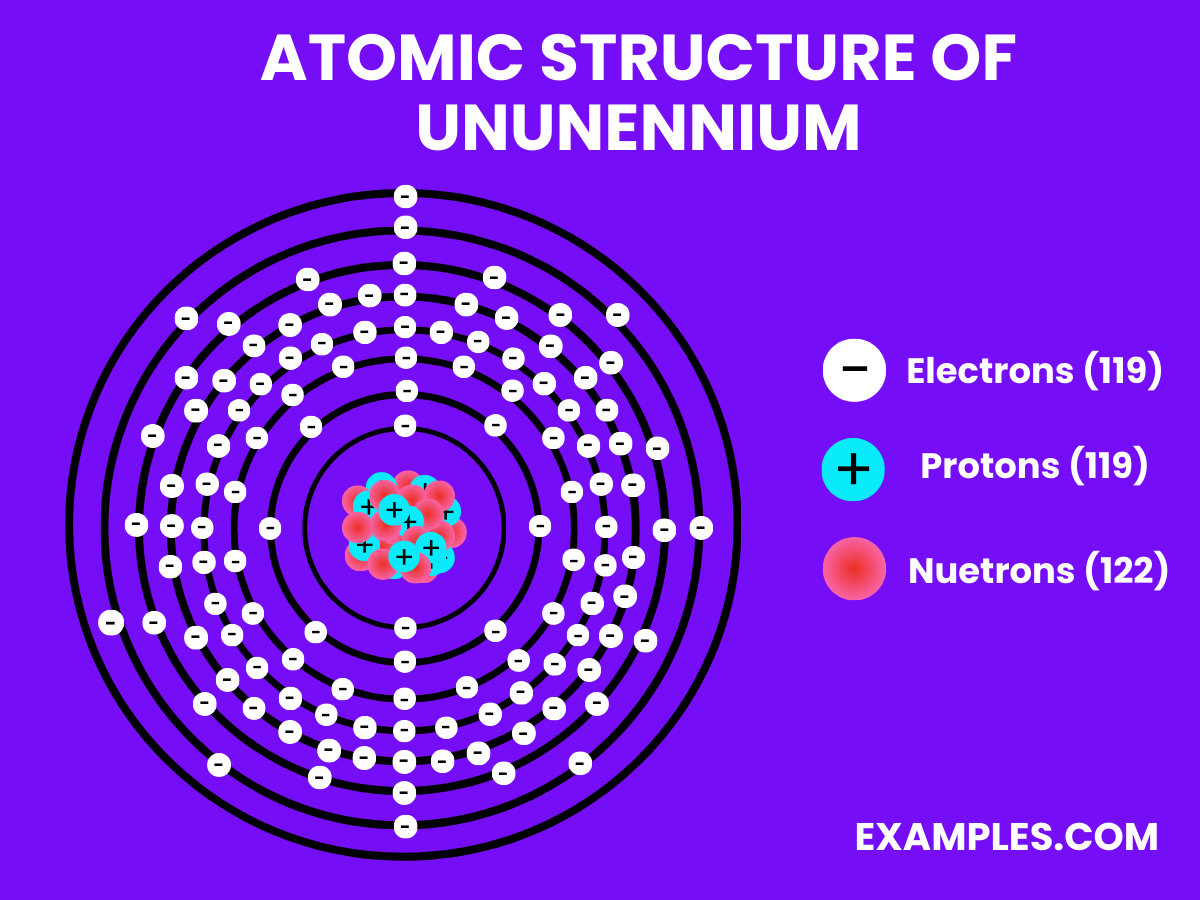
The structure of Ununennium, an element with the periodic number 119, remains a topic of scientific speculation, as it has not yet been discovered or synthesized. Based on its position in the periodic table, ununennium is predicted to be the next alkali metal, following the properties and structure trends of its group members like lithium, sodium, and potassium. These elements are characterized by having a single electron in their outermost shell, making them highly reactive. Therefore, ununennium is expected to have a similar electronic configuration, with a single valence electron contributing to its chemical behavior and reactions.
Given its theoretical status, the detailed atomic structure, including the arrangement of electrons, protons, and neutrons, is yet to be defined through experimental observation and confirmation. The anticipation around ununennium’s structure and properties fuels ongoing research, as scientists worldwide aim to synthesize this element, shedding light on the unknown aspects of the periodic table’s eighth row. This endeavor not only advances our understanding of atomic theory but also paves the way for new materials and technologies.

Here’s a table that outlines the theoretical physical properties of ununennium, drawing on trends observed in its group:
| Property | Expected Characteristics of Ununennium |
|---|---|
| State at Room Temperature | Likely to be a solid, based on the states of other alkali metals at room temperature. |
| Color | Predicted to be silvery-white or metallic, similar to other alkali metals. |
| Density | Expected to be higher than that of francium, the heaviest alkali metal currently known. |
| Melting Point | Anticipated to have a low melting point, characteristic of alkali metals, but exact value is unknown. |
| Boiling Point | Likely to have a relatively low boiling point, following the trend in its group, though the specific temperature is speculative. |
| Electrical Conductivity | Predicted to be a good conductor of electricity, akin to other members of its group. |
| Reactivity | Highly reactive, especially with water, as is typical for alkali metals. The exact reactivity level is yet to be determined. |
When ununennium comes into contact with water, it is expected to react vigorously, producing ununennium hydroxide and hydrogen gas. This reaction can be represented as follows:
Uue + 2 H₂O → Uue(OH)₂ + H₂↑
This equation suggests an intense reaction, highlighting ununennium’s position as an extremely reactive element, even more so than cesium or francium, the current last known alkali metals.
Upon exposure to oxygen, ununennium is predicted to form an oxide. The reaction might be similar to that of other alkali metals with oxygen, potentially forming ununennium oxide (UueO₂), as demonstrated in the equation below:
4 Uue + O₂ → 2 UueO₂
This reaction emphasizes the alkali metal characteristic of forming oxides upon reacting with oxygen, although the exact stoichiometry might vary until experimentally confirmed.
Ununennium is expected to readily react with halogens to form various halides. For instance, its reaction with fluorine would likely produce ununennium fluoride (UueF), as shown in the following equation:
2 Uue + F₂ → 2 UueF
Similar reactions are anticipated with other halogens (chlorine, bromine, iodine), following the general trend of forming ionic bonds characteristic of alkali metals reacting with halogens.
While an equation does not directly represent ionization energy, it is a critical factor indicating ununennium’s reactivity. The low ionization energy of ununennium would make it easy for the element to lose its outermost electron, as suggested by its position in the periodic table, enhancing its reactivity in forming compounds.
Electronegativity does not have a direct chemical equation, but it significantly influences how ununennium would behave in chemical reactions. Its very low electronegativity would mean ununennium atoms have a minimal attraction for electrons in a chemical bond, making it likely to donate its electron in reactions and form positively charged ions (cations).
These equations and descriptions provide a theoretical insight into ununennium’s chemical behavior, underlining its expected position as a highly reactive alkali metal.
| Thermodynamic Property | Theoretical Expectation for Ununennium |
|---|---|
| Standard State | Solid (assumed based on its group’s characteristics at room temperature) |
| Melting Point | Likely low, following the trend of decreasing melting points in the group |
| Boiling Point | Expected to be relatively low, consistent with alkali metals’ trends |
| Heat of Fusion | Anticipated to be moderate, as seen in other alkali metals, but unknown |
| Heat of Vaporization | Predicted to follow the trend of alkali metals, yet remains speculative |
| Specific Heat Capacity | Likely to be high, in line with the general properties of alkali metals |
| Thermal Conductivity | Expected to be good, similar to other members of its group |
| Material Property | Expected Characteristics for Ununennium |
|---|---|
| State at Room Temperature | Predicted to be solid, mirroring the physical state of other alkali metals. |
| Hardness | Likely to be soft, as alkali metals are known for their low hardness. |
| Tensile Strength | Expected to have low tensile strength, consistent with the group’s properties. |
| Ductility | Anticipated to be ductile, allowing it to be stretched into thin wires. |
| Malleability | Likely to be highly malleable, easily deformed under pressure without breaking. |
| Corrosion Resistance | Expected to be highly reactive and not resistant to corrosion, especially in the presence of water or air. |
| Thermal Expansion | Predicted to have a high thermal expansion rate, similar to other alkali metals. |
| Electrical Conductivity | Expected to be an excellent conductor of electricity, following the trend of its group. |
| Property | Expected Characteristic |
|---|---|
| Electrical Conductivity | Anticipated to be very high, as ununennium is expected to be a metal, allowing electrons to flow freely. |
| Magnetic Susceptibility | Likely to be paramagnetic or diamagnetic, similar to other alkali metals, depending on its electronic structure. |
| Ionization Energy | Expected to be low, facilitating the loss of its outer electron and enhancing its conductivity. |
| Electronegativity | Predicted to have very low electronegativity, indicating a weak ability to attract electrons in a bond. |
| Property | Expected Characteristic |
|---|---|
| Mass Number | Predicted to start around 295, considering the most stable isotopes of other superheavy elements. |
| Stability | Likely to have a short half-life, characteristic of superheavy elements, due to rapid radioactive decay. |
| Decay Modes | Expected to undergo alpha decay or possibly spontaneous fission, similar to other heavy alkali metals. |
| Magic Numbers | Though not a magic number itself, its pursuit involves reaching closer to theorized “island of stability” numbers. |
| Neutron Capture | May exhibit slow or fast neutron capture, impacting its potential stability and synthesis process. |
Selection of Target and Projectile: Scientists would choose a heavy element, such as bismuth (Bi) or lead (Pb), as a target material. They would then select a lighter element, like calcium (Ca), as the projectile, due to its 20 protons adding up to ununennium’s 119 when combined with the target.
Acceleration: The projectile ions are accelerated to high speeds using a particle accelerator. This equipment boosts the ions to the necessary velocities for overcoming the repulsive forces between two nuclei.
Collision: The accelerated ions collide with the target material in a specially designed detector chamber. The energy and speed of these collisions must be precisely controlled to encourage the nuclei of the target and projectile atoms to fuse rather than shatter.
Detection of Synthesis: If fusion occurs, a new atom of ununennium would be formed momentarily before it decays. Special detectors track the radiation signatures of these decay events, indicating the creation of the new element.
Confirmation: The detection of specific decay patterns, unique to ununennium, would confirm its synthesis. Researchers would repeat the experiment to ensure reproducibility, a key criterion for official recognition by the scientific community.
Ununennium hydride is predicted to form when ununennium reacts with hydrogen, similar to other alkali metals forming hydrides. This compound would likely be highly reactive and unstable.
Equation: Uue + H₂ → UueH₂
Ununennium oxide would be expected if ununennium reacts with oxygen. This compound might exhibit properties similar to the oxides of other alkali metals, possibly forming a basic oxide.
Equation: 4 Uue + O₂ → 2 UueO₂
This compound is anticipated to result from the reaction of ununennium with fluorine, forming a ununennium salt that is likely highly soluble in water.
Equation: Uue + F₂ → UueF₂
Ununennium chloride would form through the reaction of ununennium with chlorine. Like other alkali metal chlorides, it would be expected to be a highly reactive salt.
Equation: Uue + Cl₂ → UueCl₂
This compound would likely result from ununennium’s reaction with iodine, forming a salt. It’s expected to share characteristics with other alkali metal iodides, being highly reactive.
Equation: Uue + I₂ → UueI₂
| Isotope | Predicted Half-life | Decay Mode |
|---|---|---|
| Uue-295 | Very short (milliseconds to seconds) | Alpha decay |
| Uue-296 | Very short (milliseconds to seconds) | Alpha decay, possibly spontaneous fission |
| Uue-297 | Very short (milliseconds to seconds) | Alpha decay |
| Uue-298 | Very short (milliseconds to seconds) | Alpha decay, possibly spontaneous fission |
| Uue-299 | Very short (milliseconds to seconds) | Alpha decay |

The primary use of ununennium, once synthesized, would be in scientific research. Its discovery would provide invaluable insights into the chemical and physical properties of superheavy elements, expanding our understanding of the periodic table and atomic theory.
Ununennium’s isotopes might offer new pathways in nuclear physics, especially concerning the stability of superheavy elements. Studying its decay modes and half-lives could help scientists explore the theoretical “island of stability” in the superheavy element region.
Although highly speculative, compounds of ununennium could have unique properties that might find applications in creating new materials. These could be of interest in fields requiring extreme conditions, such as high-energy physics experiments.
If ununennium or its isotopes exhibit any unique radioactive properties, they might find niche applications in medicine or industry, similar to how other radioactive elements are used in cancer treatment or in radiographic equipment.
The discovery and study of ununennium would serve as a powerful educational tool, illustrating the complexities of chemical synthesis, the periodic law, and the limits of chemical knowledge, inspiring future generations of scientists.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of Ununennium?
118
119
120
121
The synthesis of Ununennium would likely involve bombarding a target nucleus with what type of particle?
Protons
Neutrons
Alpha particles
Heavy ions
In which part of the periodic table would Ununennium be placed?
Between Hydrogen and Helium
Between Radon and Francium
After Oganesson
Before Lithium
What is the primary challenge in detecting Ununennium?
Its inertness
Its high density
Its extremely short half-life
Its non-radioactive nature
Which is the heaviest element currently known that precedes Ununennium in the periodic table?
Livermorium
Oganesson
Tennessine
Flerovium
What is the expected oxidation state of Ununennium in its compounds?
-1
0
+1
+2
Which of the following is a predicted property of Ununennium?
High electronegativity
Low reactivity
High reactivity
Non-metallic nature
In which phase is Ununennium expected to exist at room temperature?
Solid
Liquid
Gas
Plasma
Which of the following is a challenge in studying Ununennium?
Its high abundance
Its non-reactive nature
Its short half-life
Its stable isotopes
Ununennium is part of which block in the periodic table?
s-block
p-block
d-block
f-block
Before you leave, take our quick quiz to enhance your learning!

