What is the atomic number of xenon?
52
54
56
58
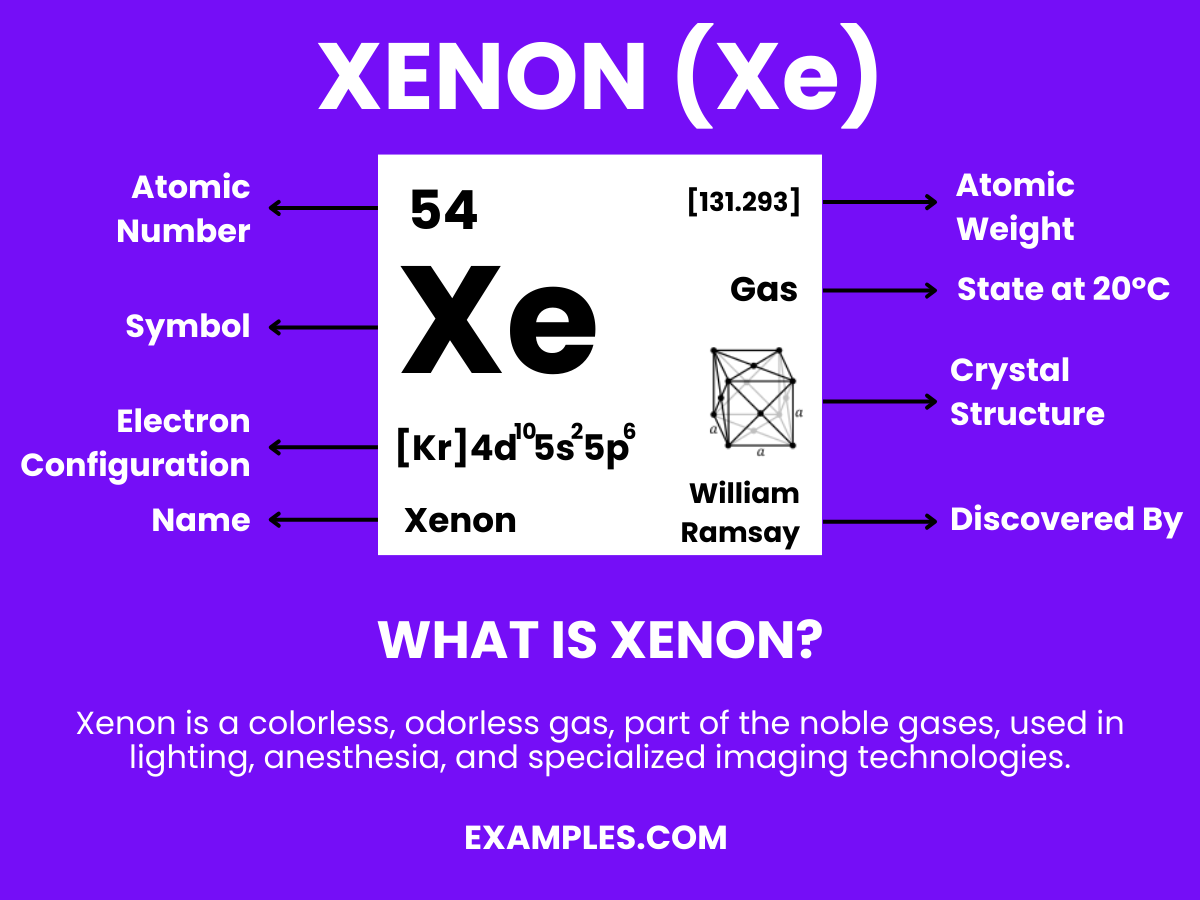
Xenon, a noble gas known for its rare and inert nature, illuminates the world in ways beyond just bright lights. As a teacher, understanding Xenon can enrich your science curriculum with fascinating examples of its uses in lighting, medical imaging, and even propulsion systems. Dive into this complete guide to discover the intriguing world of Xenon, its properties, and its applications. Engage students with real-life examples and tips on how Xenon is revolutionizing various fields.
Xenon is a colorless, dense, odorless noble gas found in the Earth’s atmosphere in trace amounts. As a noble gas, it is chemically inert, not reacting with other elements under standard conditions. It’s widely used in light-emitting devices due to its brilliant emissions and has applications in medical imaging and satellite propulsion. Understanding Xenon is essential for exploring advanced concepts in chemistry and physics, making it a fascinating subject for educators and students alike.
| Helium |
| Neon |
| Argon |
| Krypton |
| Radon |
Formula: Xe
Composition: A single xenon atom.
Bond Type: Generally non-reactive, with a complete valence shell.
Molecular Structure: Monatomic gas.
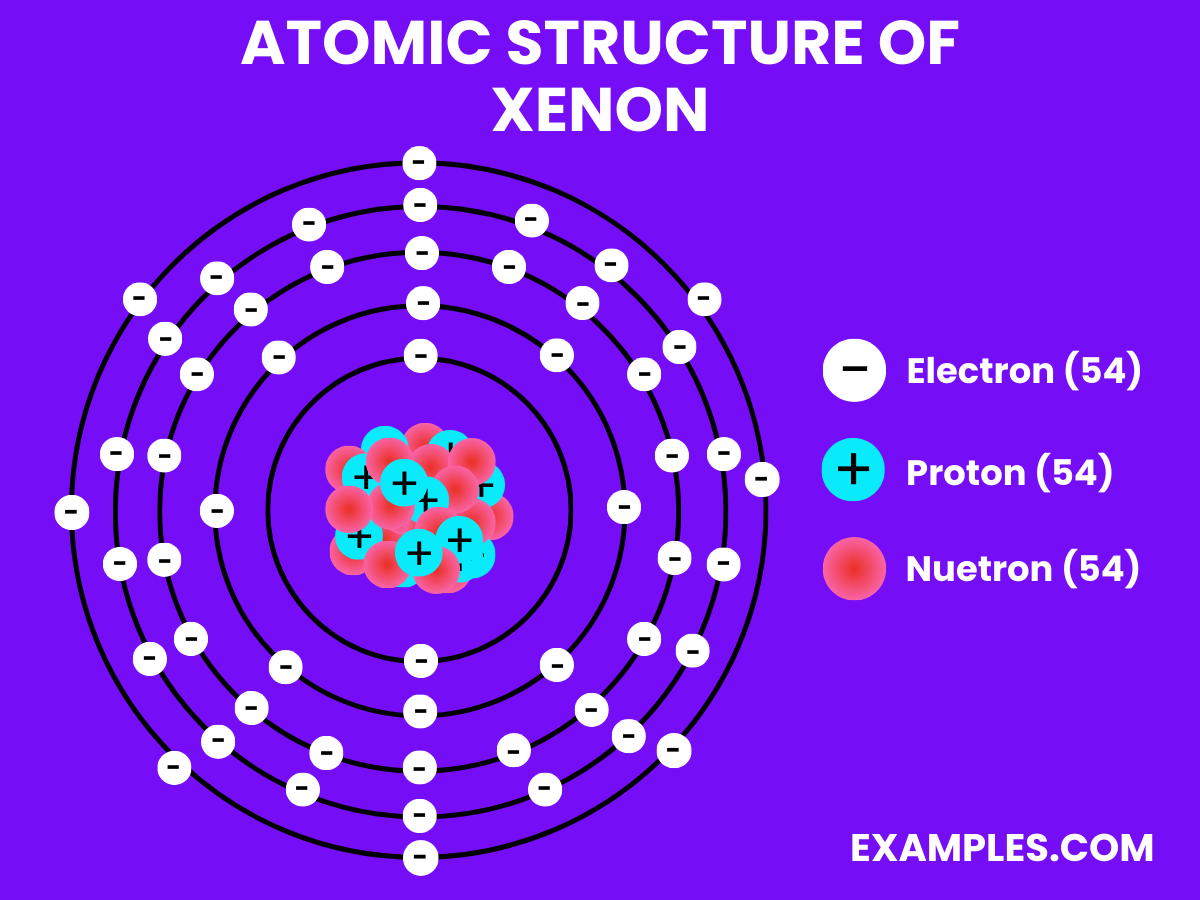
Electron Configuration: 54 electrons; configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶ 4d¹⁰ 5s² 5p⁶.
Significance: Used in lighting, like high-intensity lamps and xenon flash lamps.
Role in Chemistry: Inert, but forms compounds like xenon difluoride (XeF₂) under extreme conditions.
| Property | Description |
|---|---|
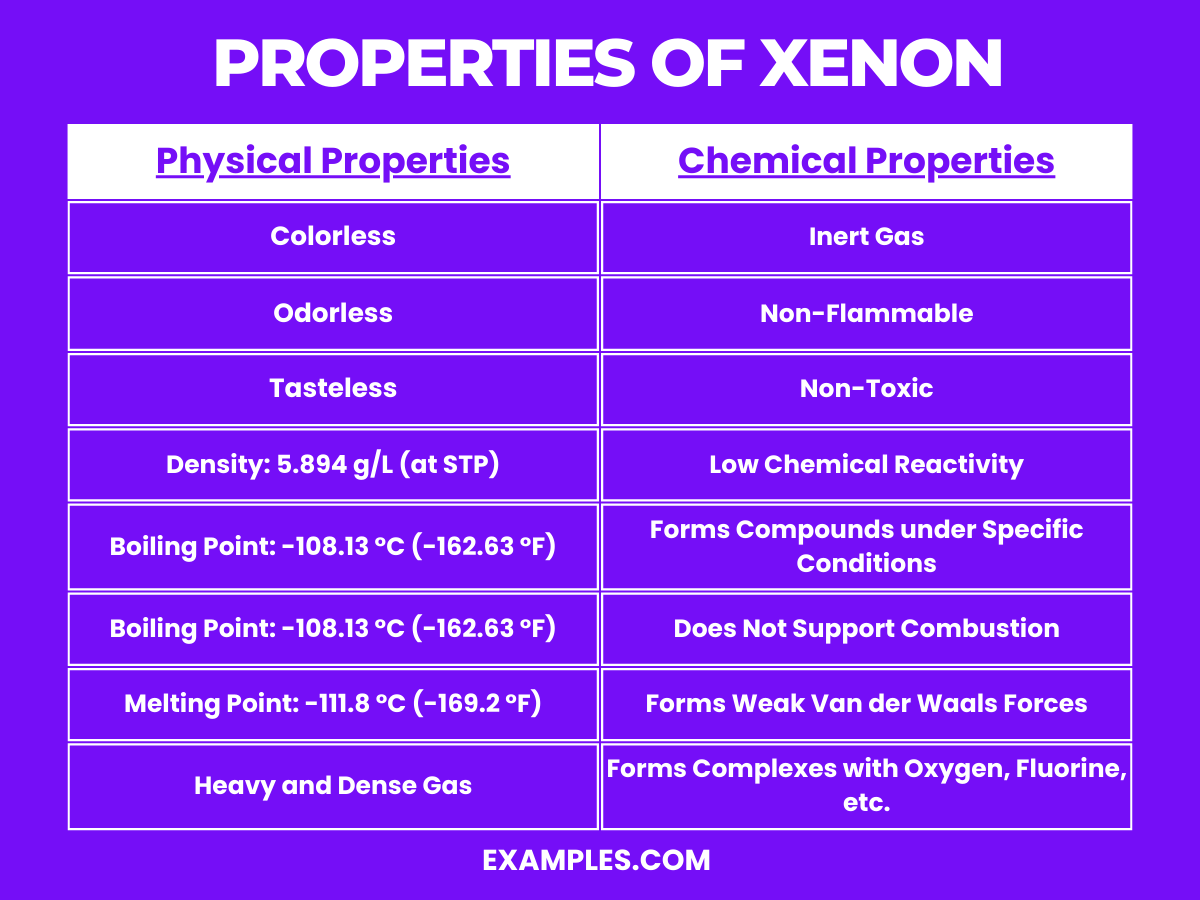
| State at Room Temperature | Gas |
| Color | Colorless |
| Odor | Odorless |
| Taste | Tasteless |
| Density | 5.894 g/L at 0°C and 1 atm |
| Boiling Point | -108.13 °C (-162.63 °F) |
| Melting Point | -111.8 °C (-169.2 °F) |
| Gas Density | Heavy and dense compared to other noble gases |
| Property | Value with Unit |
|---|---|
| Boiling Point | -108.1 °C |
| Melting Point | -111.8 °C |
| Critical Temperature | 16.6 °C |
| Critical Pressure | 5.84 MPa |
| Heat of Vaporization | 12.64 kJ/mol |
| Heat of Fusion | 2.27 kJ/mol |
| Specific Heat Capacity (at 25°C) | 0.158 J/g·K |
| Thermal Conductivity | 0.00565 W/m·K |
| Property | Value with Unit |
|---|---|
| Density (at 0°C and 1 atm) | 5.894 kg/m³ (Gas) |
| Viscosity (at 0°C) | 0.021 mPa·s (Gas) |
| Solubility in Water (at 20°C) | 0.1 g/100 mL of water |
| Phase at Room Temperature | Gas |
| Color | Colorless |
| Odor | Odorless |
| Property | Value with Unit |
|---|---|
| Electrical Conductivity | Non-conductive |
| Electronegativity (Pauling scale) | 2.6 |
| Ionization Energy | First: 12.13 eV |
| Electron Affinity | 0 eV (Practically Non-reactive) |
| Property | Value with Unit |
|---|---|
| Atomic Number | 54 |
| Atomic Mass | 131.293 amu |
| Isotopes | Numerous (stable: ^124Xe, ^126Xe, ^128Xe, ^129Xe, ^130Xe, ^131Xe, ^132Xe, ^134Xe, ^136Xe) |
| Natural Abundance (for ^129Xe) | 26.4% |
| Natural Abundance (for ^132Xe) | 26.9% |
| Nuclear Spin (for ^129Xe) | 1/2 ℏ |
| Nuclear Spin (for ^131Xe) | 3/2 ℏ |
| Neutron Cross Section (for ^129Xe) | 21 barns |
| Neutron Cross Section (for ^131Xe) | 85 barns |
| Nuclear Magnetic Moment (for ^129Xe) | -0.778 µN |
| Nuclear Magnetic Moment (for ^131Xe) | 0.692 µN |
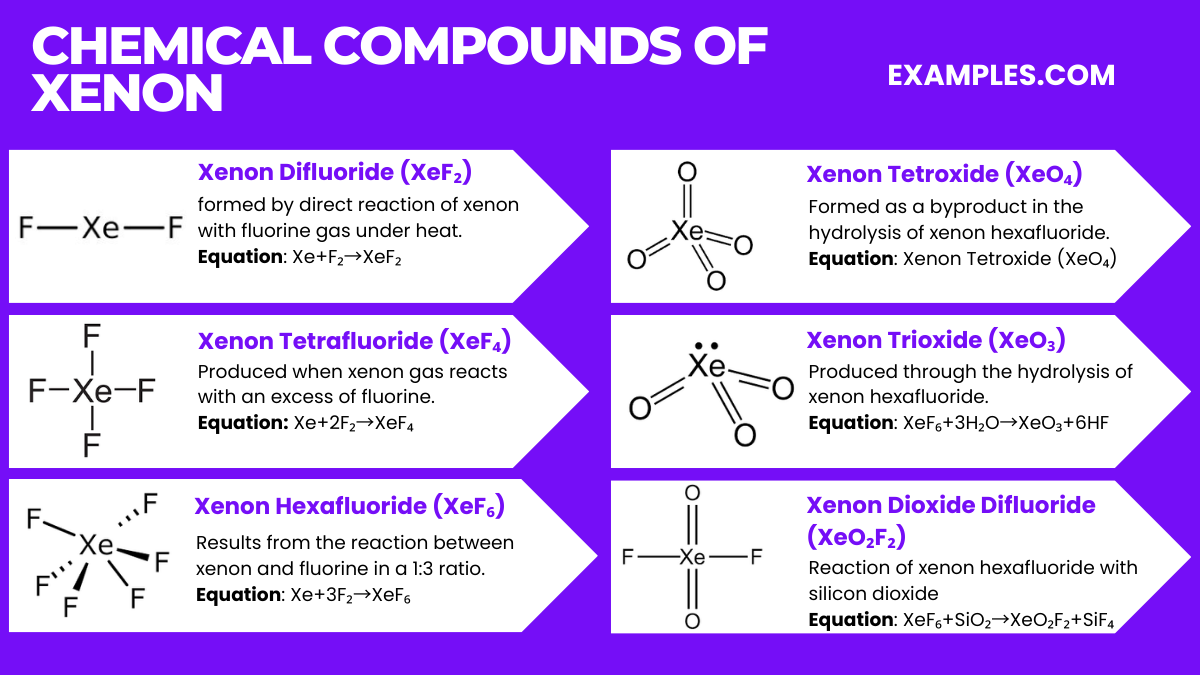
Xenon, a noble gas, forms several intriguing chemical compounds despite its general inertness. Below are the top six compounds of xenon along with their relevant equations:
Xenon has several isotopes, each with unique properties. The table below describes some of the key isotopes of xenon:
| Isotope | Mass Number | Natural Abundance (%) | Half-Life | Decay Mode |
|---|---|---|---|---|
| Xe-124 | 124 | 0.095 | 1.8 x 10²² years | Double electron capture |
| Xe-126 | 126 | 0.089 | Stable | – |
| Xe-128 | 128 | 1.92 | Stable | – |
| Xe-129 | 129 | 26.44 | Stable | – |
| Xe-130 | 130 | 4.08 | Stable | – |
| Xe-131 | 131 | 21.18 | Stable | – |
| Xe-132 | 132 | 26.89 | Stable | – |
| Xe-134 | 134 | 10.44 | Stable | – |
| Xe-136 | 136 | 8.87 | 2.165 x 10²¹ years | Double beta decay |
Xenon, a noble gas, is utilized in various applications due to its unique properties. Here are five of the most prominent uses of xenon:
The commercial production of xenon is primarily achieved through the fractional distillation of liquefied air. Here is a simplified overview of the process:
Due to the extensive processing required and the low concentration of xenon in the atmosphere, the production of xenon is relatively expensive compared to other gases. Nonetheless, its unique properties make it highly valuable for the various applications mentioned.
Xenon, as an inert, noble gas, is generally considered to be non-toxic and chemically unreactive. However, it can have certain health effects, particularly when used in medical or industrial settings:
Xenon, being a rare and inert gas, has minimal environmental impact:
Xenon is rare due to its low abundance in Earth’s atmosphere, comprising just 0.0000087%, making it one of the least common elements.
Xenon can react under certain conditions, primarily forming compounds with fluorine and oxygen, despite its general inertness as a noble gas.
Xenon glows blue when electrified because it emits light in the blue spectrum. This is due to electron excitation and subsequent release of photons.
Xenon is important for its unique applications in lighting, medical imaging, anesthesia, space exploration, and nuclear energy research, leveraging its inert and dense properties.
Xenon, a rare and noble gas, plays a crucial role in diverse fields due to its unique properties. From enhancing lighting technology to its use in medical imaging and anesthesia, xenon’s applications highlight its importance. Understanding xenon’s characteristics and reactions offers valuable insights, beneficial for industries and scientific research alike.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What is the atomic number of xenon?
52
54
56
58
Xenon is a:
Metal
Metalloid
Noble gas
Non-metal
Which of the following is a common use of xenon?
In light bulbs
As a structural material
In chemical synthesis
As a catalyst
Xenon was discovered in which year?
1889
1898
1905
1912
What is the primary method of obtaining xenon?
Mining
Fractional distillation of air
Chemical synthesis
Electrolysis of water
Which of the following properties is NOT true for xenon?
It is a good conductor of electricity
It is colorless
It is odorless
It is heavy
Xenon is most commonly found in:
The Earth\'s crust
The Earth\'s atmosphere
The ocean
The human body
Which of the following isotopes of xenon is used in medical imaging?
Xenon-129
Xenon-131
Xenon-134
Xenon-136
Xenon reacts with fluorine to form:
Xenon difluoride
Xenon tetrafluoride
Xenon hexafluoride
All of the above
Xenon is a:
Solid at room temperature
Liquid at room temperature
Gas at room temperature
Plasma at room temperature
Before you leave, take our quick quiz to enhance your learning!

