Beer-Lambert Law
What is Beer-Lambert Law Statement?
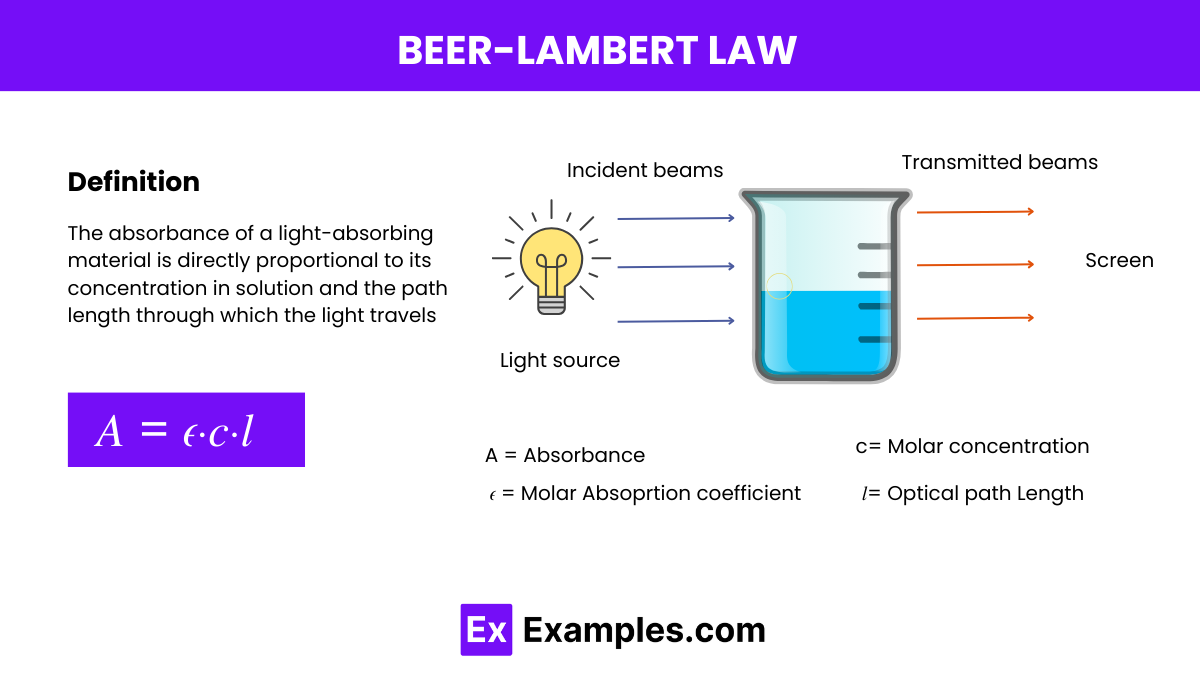
The Beer-Lambert Law, also known simply as Beer’s Law, is a fundamental statement in spectroscopy that relates the absorption of light to the properties of the material through which the light is traveling. The law states:
This law provides a quantitative basis for analyzing the concentration of an absorbing species in solution by measuring how much light is absorbed at a specific wavelength as it passes through a given path length of the solution.
What Is Beer’s Law?
Beer’s Law, also known as the Beer-Lambert Law, is a fundamental relationship that describes the absorption of light by a substance in solution. It quantitatively establishes how the absorption of light by a medium is directly proportional to the concentration of the absorbing substance and the path length of the light through that medium. The mathematical expression of Beer’s Law is:
- A is the absorbance (no units, as it is a logarithmic measure),
𝜖 is the molar absorptivity or extinction coefficient of the substance - c is the concentration of the substance in the solution
- l is the path length that the light travels through the solution (in centimeters).
What Is Lambert Law?
Lambert’s Law, also known as Lambert-Beer Law or simply Beer’s Law when combined with Beer’s formulation, is a principle in optics and spectroscopy. It states that the absorbance of light by a substance in solution is directly proportional to the concentration of the substance and the path length of the light through the solution. The formula for Lambert’s Law is:
Formula of Beer-Lambert Law
The formula for the Beer-Lambert Law, which describes the relationship between the absorbance of light by a solution and the properties of that solution, is given by:
- A is the absorbance, a unitless measure derived from the logarithm of the ratio of incident light to transmitted light.𝜖 (epsilon) is the molar absorptivity or extinction coefficient, expressed in units of
𝐿⋅𝑚𝑜𝑙−1⋅𝑐𝑚−1L⋅mol−1⋅cm−1. It represents the absorbance of a solution at a concentration of 1 mole per liter and a path length of 1 cm. - c is the concentration of the absorbing species in the solution, measured in moles per liter (𝑚𝑜𝑙⋅𝐿−1mol⋅L−1).
- l is the path length that the light travels through the solution, measured in centimeters (cm).
This formula allows you to calculate the absorbance of light by a solution, providing a means to determine the concentration of an unknown sample based on its light absorption properties.
Examples of Beer-Lambert Law
The Beer-Lambert Law is extensively used in chemistry, biology, and physics to determine the concentration of absorbing species in solution. Here are several practical examples of its application:
Concentration Determination in Chemistry
One of the most common applications of the Beer-Lambert Law is in analytical chemistry for determining the concentration of a chemical species in solution. By measuring the absorbance at a known wavelength, chemists can use the formula to calculate the concentration of the solution. This is particularly useful in titrations, where the concentration of an unknown solution is determined by comparing its absorbance to a standard curve of known concentrations.
Medical Diagnostics
In medical diagnostics, the Beer-Lambert Law is used to measure blood oxygen levels and other biochemical components. For instance, pulse oximetry, a non-invasive method that measures the oxygen saturation level of the blood, relies on the principles of this law by analyzing the absorption of light through capillary blood at different wavelengths.
Environmental Monitoring
Environmental scientists use the Beer-Lambert Law to monitor water quality by measuring the concentration of pollutants. Spectrophotometry, which is based on this law, helps in detecting and quantifying substances like heavy metals and organic compounds in water samples.
Food and Beverage Industry
The law is also applied in the food and beverage industry to determine the concentration of colorants and additives in products. For example, the concentration of food dyes can be quantified by measuring the absorbance of light passing through a solution of the dye.
Pharmaceutical Applications
In pharmaceutical development and quality control, the Beer-Lambert Law is utilized to assess the purity and concentration of solutions. Measuring the absorbance of light by drug solutions helps in ensuring that each batch meets the required standards.
Biological Research
Biologists apply this law to measure concentrations of different biomolecules in research. For example, the quantification of protein concentrations using spectrophotometric assays like the Bradford protein assay often involves principles derived from the Beer-Lambert Law.
Spectrophotometry
Quantifying the concentration of a solute in a solution by measuring the absorbance of light at a specific wavelength.
Pharmaceutical analysis
Assessing the concentration of drugs in pharmaceutical formulations for quality control purposes.
These examples demonstrate the versatility and critical importance of the Beer-Lambert Law across various scientific disciplines and industries.
Limitations of Beer-Lambert Law
1.Chemical Interactions: The law assumes that the absorbing species are independent of each other and do not interact. However, in real-world scenarios, interactions such as dimerization, association, or dissociation can occur, affecting the absorbance and leading to deviations from the expected linear relationship.
2.High Concentration Effects : At high concentrations, the solute molecules can be close enough to affect each other’s absorption properties. This can lead to deviations due to changes in refractive index, molecular aggregation, or reabsorption of light within the medium. The Beer-Lambert Law assumes that absorbance is linearly related to concentration, which may not hold true at higher concentrations.
3.Stray Light : Stray light in the spectrophotometer can cause errors in the measurement of absorbance, particularly at high absorbance values where the transmitted light is very low. This effect can lead to non-linear responses that violate the assumptions of the Beer-Lambert Law.
4.Polychromatic Light: The law is ideally applied under conditions where monochromatic light (light of a single wavelength) is used. If polychromatic light (light containing multiple wavelengths) is used, each component of the light might be absorbed differently, leading to inaccuracies unless corrections are made.
5.Scattering of Light : If the solution contains particles that can scatter light (such as a colloidal suspension), the scattered light can contribute to the apparent absorbance. This effect is particularly significant in biological samples, which often contain particles that scatter light.
6.Path Length Variations : The law assumes a uniform path length for all light rays passing through the sample. In practice, irregularities in the cuvette (container) or the positioning of the sample can lead to variations in path length, affecting the accuracy of the absorbance measurement.
7.Non-homogeneous Samples : Inhomogeneities within the sample, such as temperature gradients or concentration variations, can also affect the absorbance measurements, leading to errors if the sample does not have uniform properties throughout.
FAQ’s
How is Beer-Lambert law used in spectroscopy?
The Beer-Lambert law is used to measure and analyze the concentration of substances by assessing the light absorbance of solutions at specific wavelengths in spectroscopy.
Why is the Beer-Lambert law important?
The Beer-Lambert law is crucial for quantitative analysis in chemistry and biology, providing a direct method for determining the concentrations of absorbing substances in solutions.
What are the three factors that affect the Beer-Lambert law?
Three key factors that influence the Beer-Lambert law are the concentration of the solution, the path length of the light through the solution, and the wavelength of the light used.