Who is J.J. Thomson?
A famous chemist
The discoverer of the electron
The developer of the periodic table
The first person to split the atom

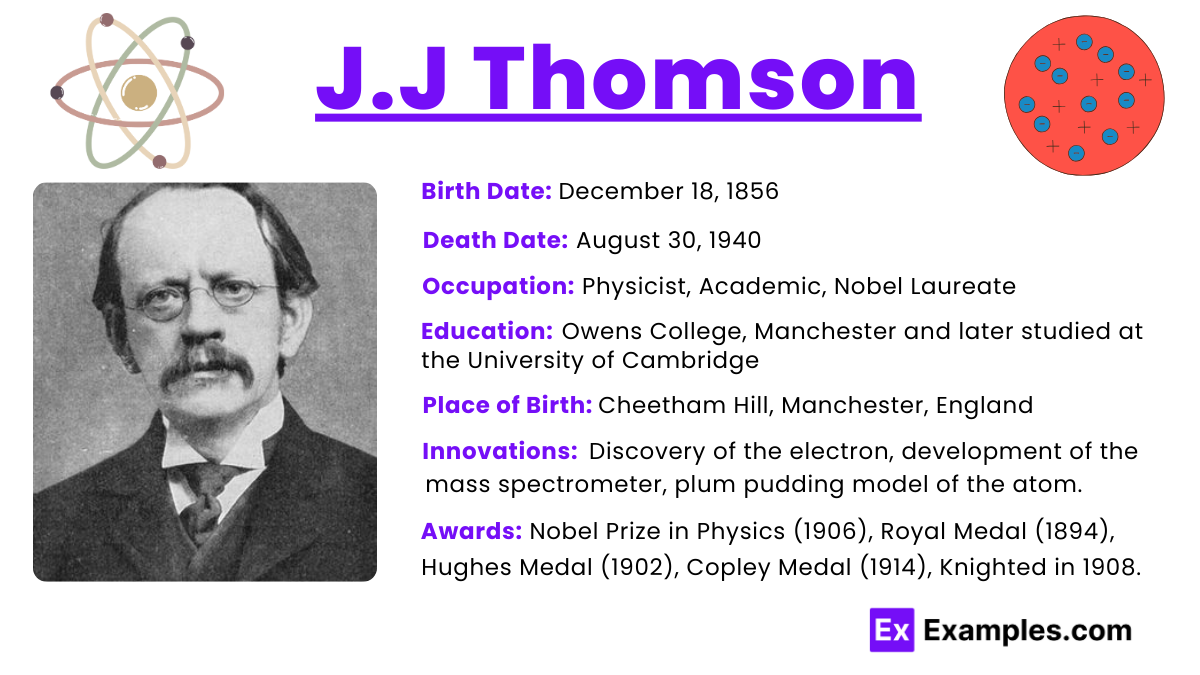
J.J. Thomson, born Joseph John Thomson in 1856, was a British physicist renowned for his discovery of the electron in 1897. Working at the Cavendish Laboratory at the University of Cambridge, Thomson demonstrated through his experiments with cathode rays that atoms are not indivisible as previously thought, but contain smaller particles. This groundbreaking work led him to identify the first subatomic particle—the electron, a negatively charged component considerably lighter than an atom. His discovery fundamentally changed the understanding of atomic structure and led to further developments in nuclear physics. For his contributions to science, Thomson was awarded the Nobel Prize in Physics in 1906, and he is also credited with developing the mass spectrometer and formulating the Thomson atomic model.
Early Years and Family Background
Joseph John Thomson, known as J.J. Thomson, was born on December 18, 1856, in Cheetham Hill, a suburb of Manchester, England. His parents were Joseph James Thomson, a bookseller and publisher, and his wife, Emma Swindells, who came from a family that owned a cotton spinning business. Despite being raised in a modest environment, his father’s influence nurtured an early interest in science and learning. The unexpected death of his father, however, nearly forced Thomson to abandon his education for a more practical apprenticeship.
Schooling Challenges and Academic Promise
Thomson began his formal education at a small private school where he showed early promise, particularly in mathematics. He later attended Owens College, Manchester (now the University of Manchester), at the age of 14. This was unusually young, and he faced challenges adjusting to the more rigorous academic environment. Nevertheless, his aptitude for science and mathematics quickly became apparent, setting the stage for his future academic pursuits.
Relocation to Cambridge and a New Direction
In 1876, Thomson was awarded a scholarship to attend Trinity College, Cambridge. This transition marked a significant change in his life and academic career. At Cambridge, he studied mathematics but gradually became more interested in experimental physics. His time at the Cavendish Laboratory under the mentorship of Lord Rayleigh would significantly influence his future research and achievements.
Graduation and Groundbreaking Research
Thomson excelled at Cambridge and was elected a Fellow of Trinity College in 1880, following his graduation. He became Cavendish Professor of Physics at the age of 28, one of the youngest to hold this prestigious position. His research during these years laid the groundwork for the discovery of the electron, which would come later in his career.
Personal Life and Later Years
In 1890, Thomson married Rose Elisabeth Paget, the daughter of Sir George Edward Paget, a physician and then Regius Professor of Physic at Cambridge. They had two children, a son, George Paget Thomson, who would also become a Nobel Prize-winning physicist, and a daughter, Joan Paget Thomson. His marriage brought stability and personal happiness, which supported him throughout his demanding career. Thomson’s home life was closely intertwined with the University of Cambridge, where he spent nearly his entire professional life, mentoring a generation of physicists who would go on to make significant contributions to the field.
J.J. Thomson was born into a modest family in Cheetham Hill, Manchester, England. His father, Joseph James Thomson, was a bookseller and publisher, which provided a cultured, albeit not affluent, environment. His mother, Emma Swindells, came from a family involved in the cotton industry. The family was deeply affected by the early death of Thomson’s father, which placed financial strains on them and nearly derailed Thomson’s future academic pursuits.
In his personal life, J.J. Thomson married Rose Elisabeth Paget in 1890. Rose was the daughter of Sir George Edward Paget, a well-known physician and professor at Cambridge. This connection likely helped Thomson in both his personal and professional life, offering stability and entrance into established social circles at the University of Cambridge. The couple had two children who continued their father’s legacy of intellectual pursuit. Their son, George Paget Thomson, inherited his father’s interest in physics and went on to win the Nobel Prize in Physics in 1937 for his discovery of the wave properties of the electron through electron diffraction. They also had a daughter, Joan Paget Thomson, who did not pursue a public life, and therefore, less is known about her endeavors.
After completing his education at Trinity College, Cambridge, J.J. Thomson remained at the institution, quickly rising through the academic ranks. In 1884, at the age of just 28, he was appointed Cavendish Professor of Physics at Cambridge, one of the most prestigious positions in the field. This role placed him at the helm of the Cavendish Laboratory, where he would make his most notable scientific contributions.
Thomson’s early work at the Cavendish Laboratory involved investigations into the properties of cathode rays. In 1897, through his experiments, he discovered the electron—then referred to as “corpuscles.” This discovery was revolutionary because it was the first identification of a subatomic particle, proving that atoms were divisible. Thomson demonstrated that cathode rays were streams of charged particles (electrons), which were much smaller than atoms and carried a negative charge. This discovery was a foundational pillar in the development of atomic physics.
Following his discovery of the electron, Thomson proposed a model for the atomic structure in 1904, famously known as the “plum pudding model.” This model suggested that the atom was a sphere of positive charge with negatively charged electrons embedded within it, much like raisins in a pudding. Although this model was later superseded by Ernest Rutherford’s nuclear model of the atom, it was significant for being one of the earliest models to include subatomic particles.
Thomson’s research also extended into the development of mass spectrometry. In 1913, building on his work with cathode rays and the discovery of isotopes by his student Frederick Soddy, Thomson designed the first mass spectrometer. This device, initially called a parabola spectrograph, was capable of separating charged particles of different masses and led to the development of the mass spectroscopic method used widely in chemistry and physics for analyzing and identifying material compositions.
The discovery of the electron by J.J. Thomson in 1897 marked a pivotal moment in the history of science. While conducting experiments at the Cavendish Laboratory in Cambridge, Thomson investigated cathode rays, which were streams of particles emitted by an electron gun in a high-vacuum tube. Through his experiments, he determined that these rays were made up of particles much smaller than atoms, which he initially termed “corpuscles.” This contradicted the prevailing notion that atoms were the smallest indivisible components of matter. By measuring the deflection of the particles in magnetic and electric fields, Thomson was able to calculate their mass and charge, conclusively proving that electrons were indeed universal components of atoms. This groundbreaking discovery not only established the existence of subatomic particles but also laid the foundation for modern atomic and quantum physics, significantly altering our understanding of the fundamental structure of matter.
J.J. Thomson’s work on isotopes and mass spectrometry began with his foundational discovery of the electron, but it was his subsequent experiments that truly expanded our understanding of atomic structure. After the discovery of the electron, Thomson’s focus shifted toward investigating the nature of ions formed in gases that were subjected to electrical discharges. This research led him to explore the composition of ion streams using an improved version of the cathode ray tube.
In 1913, Thomson developed one of the earliest forms of mass spectrometry, which he initially called a “parabola spectrograph” because of the parabolic paths that ions followed in the magnetic and electric fields of his apparatus. This instrument was crucial for separating and measuring the mass-to-charge ratio of ionized atoms and molecules, enabling Thomson to demonstrate that neon gas was composed of atoms of two different atomic masses. This was the first clear evidence that elements could exist as isotopes, or atoms of the same element that have different numbers of neutrons and consequently different mass numbers.
J.J. Thomson’s experiments with cathode rays were instrumental in his groundbreaking discovery of the electron in 1897, a fundamental step forward in the field of atomic physics. At the Cavendish Laboratory at the University of Cambridge, Thomson conducted a series of experiments using cathode ray tubes, which are glass tubes evacuated of air and fitted with two electrodes to emit rays from the cathode (negative electrode) to the anode (positive electrode).
In his experiments, Thomson passed the cathode rays through electric and magnetic fields, observing their behavior under these influences. By measuring the degree of deflection of the rays as they passed through these fields, Thomson could deduce several important properties of the particles in the rays, such as their charge and mass. His experiments revealed that the particles were deflected by the magnetic and electric fields in a manner consistent with particles that were much smaller than atoms and negatively charged.
The Plum Pudding Model, proposed by J.J. Thomson in 1904, was his conceptual model of the atom, developed shortly after his discovery of the electron. In this model, Thomson envisioned the atom as a sphere of positive charge with negatively charged electrons embedded within it, much like raisins in a plum pudding (a popular British dessert). This arrangement was hypothesized to balance the positive and negative charges, making the atom electrically neutral. Although it was eventually superseded by Ernest Rutherford’s nuclear model of the atom, which introduced a central nucleus, Thomson’s Plum Pudding Model was crucial as it incorporated the newly discovered electrons into the structure of the atom and suggested that atoms were divisible, laying the groundwork for future atomic models and advancing the understanding of atomic structure during the early 20th century.
In 1906, J.J. Thomson was awarded the Nobel Prize for his discovery of the electron and research on the conduction of electricity through gases.
J.J. Thomson did not discover a new type of ray; he studied cathode rays, determining they were streams of negatively charged particles later named electrons.
Thomson discovered that electricity conducted through gases in cathode ray tubes involves tiny negatively charged particles, which he identified as electrons.
J.J. Thomson conducted his experiments to investigate the nature of cathode rays and to understand the fundamental components of atoms.
J.J. Thomson discovered the electron, a fundamental subatomic particle carrying a negative charge, pivotal to the development of atomic physics.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
Who is J.J. Thomson?
A famous chemist
The discoverer of the electron
The developer of the periodic table
The first person to split the atom
What device did J.J. Thomson use to discover the electron?
Mass spectrometer
Particle accelerator
Cathode ray tube
Cloud chamber
In what year did J.J. Thomson discover the electron?
1887
1897
1907
1917
What charge does an electron carry?
Positive
Negative
Neutral
Variable
What is the model of the atom proposed by J.J. Thomson called?
Planetary model
Quantum model
Plum pudding model
Rutherford model
Which of the following experiments led to the discovery of the electron?
Gold foil experiment
Oil drop experiment
Alpha particle scattering experiment
Cathode ray experiment
What is the significance of J.J. Thomson's discovery?
It proved the existence of isotopes
It led to the discovery of the nucleus
It identified the first subatomic particle
It confirmed the wave nature of particles
What prestigious award did J.J. Thomson receive for his discovery of the electron?
Nobel Prize in Physics
Copley Medal
Franklin Medal
Royal Medal
Which institution was J.J. Thomson affiliated with when he discovered the electron?
Harvard University
University of Cambridge
University of Oxford
Massachusetts Institute of Technology
What is the significance of J.J. Thomson's work on isotopes?
It demonstrated the existence of neutrons
It showed that elements can have atoms with different masses
It proved the existence of protons
It confirmed the uncertainty principle
Before you leave, take our quick quiz to enhance your learning!

