What does Planck\'s Law describe?
Motion of electrons
Distribution of electromagnetic radiation
Gravitational force between masses
Conservation of momentum

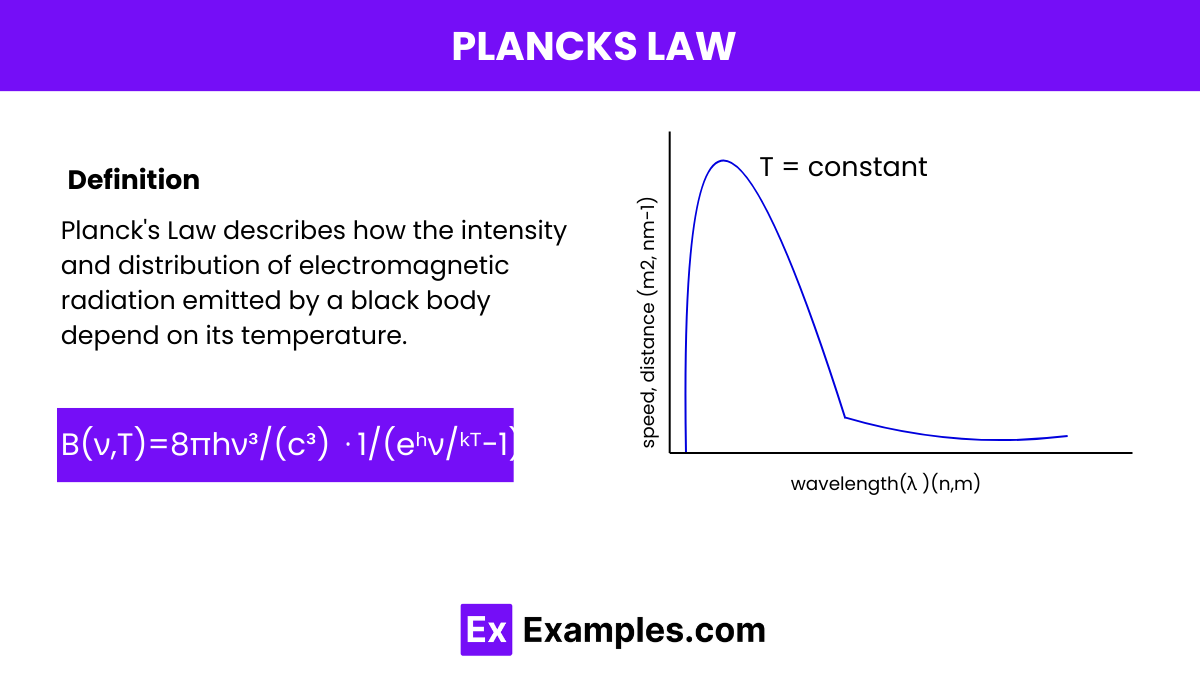
Planck’s Law can be expressed mathematically as:
Planck’s constant, denoted by ℎ, is a fundamental physical constant that plays a crucial role in quantum mechanics. It represents the proportionality factor between the energy of a photon and the frequency of its associated electromagnetic wave, as described by the equation 𝐸=ℎ𝜈, where 𝐸 is the energy of the photon and 𝜈 is its frequency. Planck’s constant has a value of approximately 6.62607015×10−³⁴ joule-seconds.
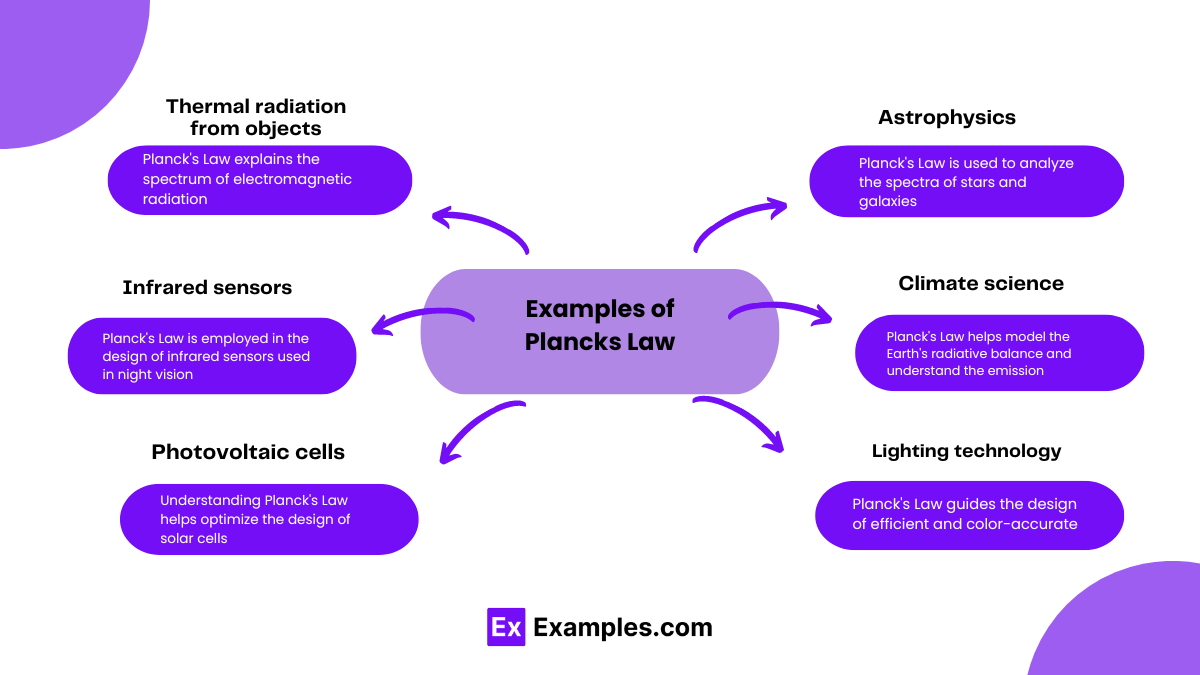
Thermal radiation from objects: Planck’s Law explains the spectrum of electromagnetic radiation emitted by objects due to their temperature. For instance, it describes the colors emitted by a hot iron rod as it heats up.
Astrophysics: Planck’s Law is used to analyze the spectra of stars and galaxies, providing insights into their temperature, composition, and evolutionary stage.
Infrared sensors: Planck’s Law is employed in the design of infrared sensors used in night vision devices, thermal imaging cameras, and remote sensing applications.
Photovoltaic cells: Understanding Planck’s Law helps optimize the design of solar cells to efficiently convert sunlight into electricity by matching their absorption spectra to the solar spectrum.
Climate science: Planck’s Law helps model the Earth’s radiative balance and understand the emission and absorption of infrared radiation by greenhouse gases, crucial for climate studies and predicting global warming.
Lighting technology: Planck’s Law guides the design of efficient and color-accurate lighting sources such as incandescent bulbs, fluorescent lamps, and light-emitting diodes (LEDs).
Medical imaging: Infrared thermography, which uses thermal radiation emitted by objects to create images, relies on Planck’s Law to interpret temperature variations in the human body for medical diagnostics.
Material characterization: Planck’s Law aids in the analysis of materials through techniques like spectroscopy, enabling researchers to identify chemical compositions and study molecular structures.
Astronomy: By applying Planck’s Law, astronomers can study cosmic microwave background radiation, the relic radiation from the early universe, to learn about the universe’s evolution and structure.
Quantum mechanics: Planck’s constant, a key component of Planck’s Law, is central to various quantum phenomena, including the quantization of energy levels in atomic and molecular systems.
While Planck’s Law is highly accurate and widely applicable, it does have some limitations:
Assumption of Black Body: Planck’s Law is derived assuming the radiation source is a perfect black body, which absorbs and emits all radiation that falls on it. In reality, few objects behave as perfect black bodies across all wavelengths.
Frequency Dependence: Planck’s Law predicts that as frequency increases, radiation intensity also increases. However, it fails to account for phenomena like the ultraviolet catastrophe, where classical physics predicted an infinite amount of high-frequency radiation.
Temperature Range: Planck’s Law is valid for a wide range of temperatures, but it may not accurately describe the behavior of radiation at extremely high temperatures or in certain extreme conditions, such as those found in astrophysical environments.
Complexity: The mathematical formulation of Planck’s Law involves complex integrals and equations, making it challenging to apply in some practical situations without specialized knowledge or computational tools.
A real-life example of Planck’s Law is the operation of incandescent light bulbs, where the color of emitted light shifts with temperature, following the predictions of Planck’s formula.
Einstein used Planck’s theory to develop the theory of photoelectric effect, for which he won the Nobel Prize in Physics in 1921.
Max Planck’s famous quote is “Science cannot solve the ultimate mystery of nature. And that is because, in the last analysis, we ourselves are part of nature and therefore part of the mystery that we are trying to solve.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What does Planck\'s Law describe?
Motion of electrons
Distribution of electromagnetic radiation
Gravitational force between masses
Conservation of momentum
What does the variable 'λ' represent in Planck's Law?
Frequency
Wavelength
Temperature
Energy
How does the spectral radiance change with increasing wavelength according to Planck's Law?
It increases indefinitely
It decreases indefinitely
It first increases then decreases
It remains constant
What does the constant 'h' stand for in the Planck's Law formula?
Planck's constant
Hubble's constant
Gravitational constant
Avogadro's number
What is the value of Planck's constant?
3.00 x 10⁸ m/s
6.63 x 10⁻³⁴ Js
1.38 x 10⁻²³ J/K
9.81 m/s²
In Planck's Law, what is 'T' a measure of?
Time
Temperature
Torque
Tension
What is the relationship between temperature and the peak wavelength in Planck's Law?
Directly proportional
Inversely proportional
Exponentially proportional
No relationship
What does 'c' represent in the Planck's Law equation?
Speed of sound
Speed of light
Capacitance
Coulomb's constant
Which physical constant is represented by 'k' in Planck's Law?
Coulomb's constant
Boltzmann's constant
Planck's constant
Stefan-Boltzmann constant
How does the spectral radiance change with increasing temperature according to Planck's Law?
It decreases
It increases
It remains the same
It fluctuates randomly
Before you leave, take our quick quiz to enhance your learning!

