What does the Rydberg formula calculate?
The speed of light
The energy levels of electrons
The wavelengths of spectral lines of hydrogen
The frequency of sound waves

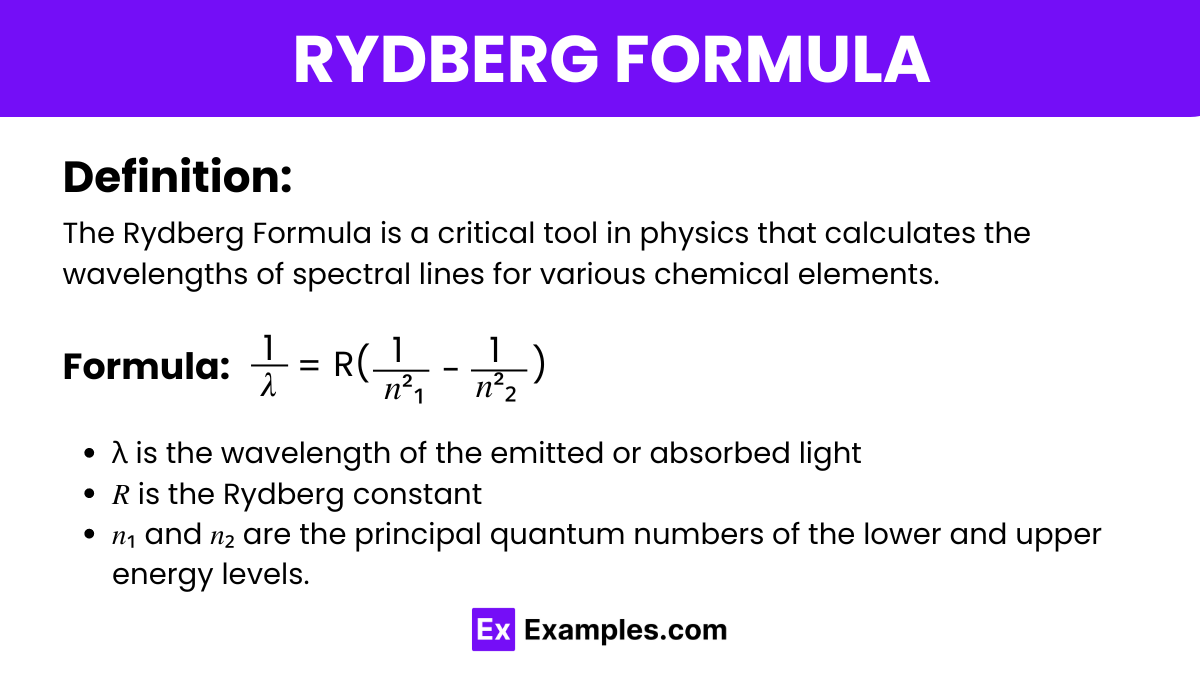
The Rydberg Formula is a critical tool in physics that calculates the wavelengths of spectral lines for various chemical elements. It specifically predicts the wavelengths of photons emitted as electrons transition between energy levels within an atom, particularly in hydrogen. This formula is instrumental in exploring atomic structures and the fundamentals of quantum mechanics, aligning closely with the laws of physics and providing insights that bridge classical and quantum theories.
The Rydberg Formula is used to calculate the wavelengths of the spectral lines of hydrogen and hydrogen-like elements. It is expressed as:
where:
This formula is crucial in spectroscopy for identifying elements and understanding atomic structure.
The derivation of the Rydberg Formula for the hydrogen atom involves understanding how energy levels in an atom relate to the frequencies (and thus wavelengths) of light emitted or absorbed when an electron transitions between these levels. Here’s a step-by-step explanation:
Step 1: Energy Levels of the Hydrogen Atom
The energy of an electron in a hydrogen atom can be expressed by the Bohr model as:
Where:
Step 2: Photon Emission and Absorption
When an electron transitions from a higher energy level 𝑛₂ to a lower energy level 𝑛₁, it emits a photon whose energy 𝐸E corresponds to the difference between these two energy levels:
Step 3: Relation to Photon Wavelength
The energy of the photon can also be related to its wavelength 𝜆 through the Planck relation:
Where:
Step 4: Combine Equations
Equating the two expressions for energy, we get:
ℎ⋅𝑐/𝜆=𝑅𝐻/𝑛²₁−𝑅𝐻/𝑛²₂λ
Rearranging for 𝜆λ, we find the Rydberg Formula for the wavelengths of emitted photons:
1/𝜆=𝑅(1/𝑛²₁−1/𝑛²₂)
Where R is the Rydberg constant for hydrogen, often used in the more general form adjusted for the specific atom by taking into account 𝑍 and Rₕ.
Here’s a table presenting the Rydberg Formula for hydrogen, the general formula for wavelength, and the values of the Rydberg constant in different units:
| Description | Formula or Value |
|---|---|
| Rydberg Formula for Wavelength | 1/𝜆=𝑅(1/𝑛²₁−1/𝑛²₂) |
| Rydberg Formula for Hydrogen | 1/𝜆=𝑅ₕ(1/1²−1/𝑛²) for n>1 |
| Rydberg Constant in cm | 1.097373×10⁵ cm⁻¹ |
| Rydberg Constant in Joules | 2.179×10⁻¹⁸ Joules (per electron volt) |
This table encapsulates the critical aspects of the Rydberg Formula and the constant, providing essential details for calculations involving atomic spectra, particularly for hydrogen.
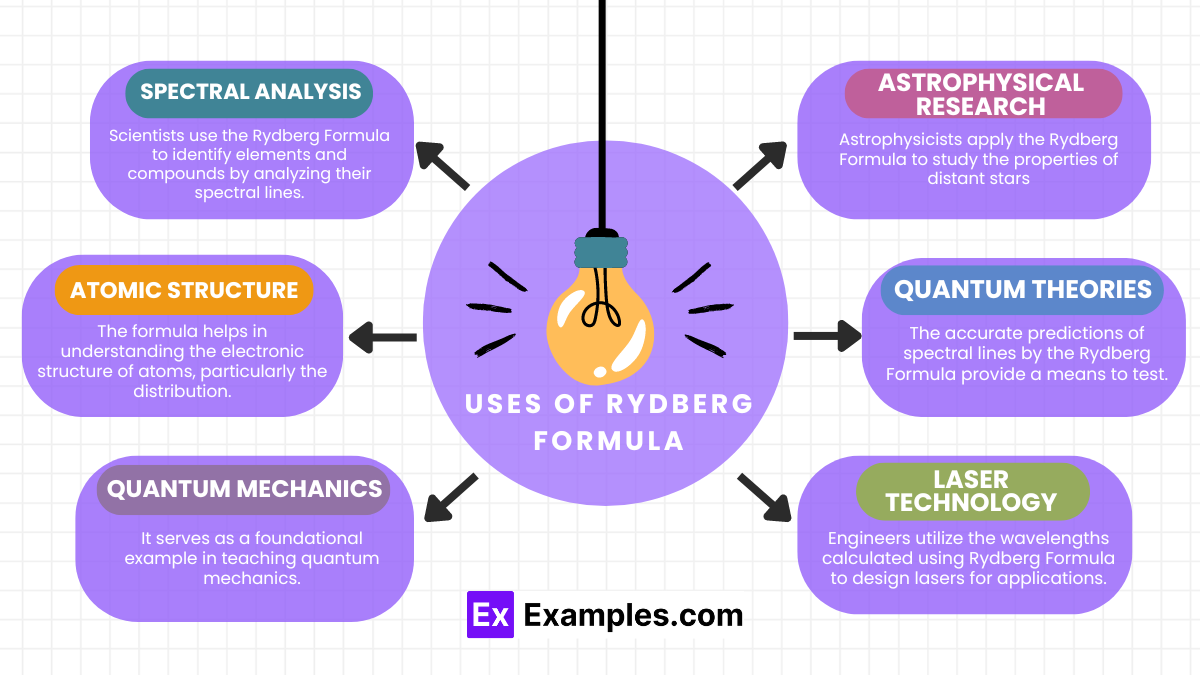
The Rydberg Formula has several important applications in physics and chemistry:
Here are some examples illustrating the practical applications of the Rydberg Formula, enriched with transition words to clarify the flow of ideas:
These examples underscore the versatility and fundamental importance of the Rydberg Formula in both theoretical and applied physics.
No, the Rydberg equation originally formulated for hydrogen can also be modified to apply to hydrogen-like (single-electron) ions of other elements.
The Rydberg equation works primarily for elements with a single electron in their outer shell, such as hydrogen-like ions, but not for multi-electron atoms.
The Rydberg equation works for hydrogen because it has one electron. For helium and other multi-electron atoms, electron-electron interactions complicate the spectrum, making the equation ineffective.
Text prompt
Add Tone
10 Examples of Public speaking
20 Examples of Gas lighting
What does the Rydberg formula calculate?
The speed of light
The energy levels of electrons
The wavelengths of spectral lines of hydrogen
The frequency of sound waves
What is the value of the Rydberg constant (Rₕ) for hydrogen?
1.097×10⁷m⁻¹
3.00×10⁸m/s
6.63×10⁻³⁴ Js
9.81 m/s².
Which of the following series corresponds to transitions where the final energy level (n₂) is 1?
Balmer series
Lyman series
Paschen series
Brackett series
In the Rydberg formula, which variable represents the principal quantum number of the initial energy level?
n₁
n₂
Rₕ
λ
For the Balmer series, what is the value of the final energy level (n₂)?
0
1
2
3
Which spectral series corresponds to transitions where the final energy level (n₂) is 3?
Balmer series
Lyman series
Paschen series
Brackett series
In the Rydberg formula, what does the variable λ represent?
Wavelength of light emitted or absorbed
Energy of the electron
Frequency of light emitted or absorbed
Velocity of the electron
What is the relationship between the wavelength (λ) and the energy difference between two energy levels in the hydrogen atom?
Directly proportional
Inversely proportional
Exponentially proportional
No relationship
In the context of the Rydberg formula, what does the variable n₂ represent?
The initial energy level
The final energy level
The wavelength of light
The speed of light
What does the Rydberg constant (R_h) represent in the Rydberg formula?
The speed of light in a vacuum
The proportionality constant for the frequency of light
The proportionality constant for the wavelength of light in the hydrogen spectrum
The mass of an electron
Before you leave, take our quick quiz to enhance your learning!

